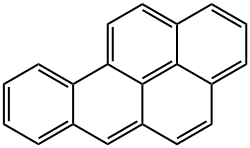
Benzo[a]pyrene:Mechanism of Toxicity
Nov 22,2021
Benzo(a)pyrene (BaP) is bioactivated to its carcinogenic form
by phase 1 and phase 2 metabolism. As with other polycyclic
aromatic hydrocarbons (PAHs), the presence of the ‘bay
region’ contributes greatly to the carcinogenicity of BaP. This
region is sterically constrained, allowing the formation of
diol epoxides, which subsequently react with intracellular
molecules such as DNA. Human exposure to Benzo(a)pyrene and other
PAHs occurs primarily from smoking or from secondhand
smoke, air polluted with combustion products, or food and
water polluted with combustion products, such as those
cooked over charcoal or broiled.
Benzo(a)pyrene has been extensively studied for its toxicities in children and during pregnancy. A study of pregnant active smokers showed that BaP crossed the human placenta and was bound to fetal hemoglobin at levels significantly higher than in pregnant nonsmokers.
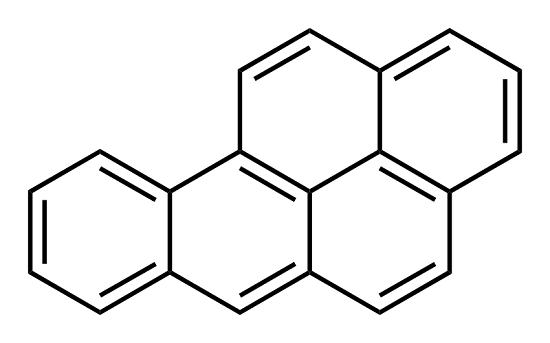
Uses
Benzo(a)pyrene is not commercially produced; it is a by-product of combustion. Its primary uses include toxicological mechanistic studies and cancer studies, as a positive control in carcinogenicity studies. There is no known commercial use for BaP.
Environmental Fate
Benzo(a)pyrene is purposely synthesized solely for laboratory studies. The primary source of BaP and many PAHs in air is the incomplete combustion of wood, gasoline, and other fuels; in industrial settings where coal is burned; and in natural burns such as forest fires. Benzo(a)pyrene can bind to particulate matter, and inhalation is a common route of exposure. BaP is poorly water soluble, partitioning strongly to the sediment, and does not readily bioaccumulate. BaP is found in fossil fuels, crude oils, shale oils, and coal tars, and is emitted with gases and fly ash from active volcanoes. If released to air, an extrapolated vapor pressure of 5.49×10-9 mm Hg at 25°C indicates BaP will exist solely in the particulate phase in the atmosphere. Particulate-phase BaP is usually removed from the atmosphere by wet or dry deposition. BaP contains chromophores that absorb at wavelengths >290 nm and therefore is expected to be susceptible to direct photolysis by sunlight; after 17 h irradiation with light >290 nm, 26.5% of BaP adsorbed onto silica gel was degraded. If released to soil, BaP is expected to have very low to no mobility based on measured soil Koc values of 930–6300. Volatilization from moist soil surfaces is not expected to be an important fate process based on a Henry’s Law constant of 4.57×10-7 atm m3 mol-1. The stability of BaP in soil is expected to vary depending on the nature of compounds accompanying it and the nature and previous history of the soil; biodegradation half-lives of 309 and 229 days were observed in Kidman and McLaurin sandy loam soils, respectively. BaP is expected to adsorb to suspended solids and sediment based on the measured Koc values, when released into water. Biodegradation of BaP is possible in aquatic systems. Volatilization from water surfaces is not expected to be an important fate process based on this compound’s Henry’s Law constant.
Mechanism of Toxicity
While not known to be toxic itself, BaP and other PAHs containing bay regions are carcinogenic. BaP is bioactivated via three enzymatic steps to create a carcinogenic epoxide resistant to epoxidases. It is first metabolized by cytochrome P450 1A1 to (+) benzo(a)pyrene 7,8-oxide, next by epoxide hydrolase to benzo(a)pyrene 7,8-dihydrodiol, and finally by cytochrome P450 1A1 to (+) benzo(a)pyrene 7,8-dihydrodiol-9,10-epoxide. The prototypical ‘bay region’ of this PAH representative facilitates DNA interaction. Other metabolites are toxic as well. The aryl hydrocarbon receptor is responsible for some BaP toxicity; knockout of AhR significantly reduced toxicity from BaP in a mouse model system.
- Related articles
- Related Qustion
- What you should know about Benzo[a]pyrene? Dec 25, 2019
Benzo[a]pyrene (B[a]P) is one of a large number of polycyclic aromatic hydrocarbons (PAHs) that are formed during the incomplete combustion of organic matter.
Ethyl 2-bromoisobutyrate, with the CAS No: 600-00-0. This chemical’s molecular formula is C6H11BrO2 and molecular Weight is 195.06g/mol. The boiling point is 161℃ at 760 mmHg.....
Nov 22,2021Chemical ReagentsCalcium hydroxide is a material which has been used for a variety of purposes since its introduction into dentistry in the early part of the twentieth century. In its pure form, the substance has a hi....
Nov 23,2021Inorganic saltsBenzo[a]pyrene
50-32-8You may like
- Benzo[a]pyrene
-
![50-32-8 Benzo[a]pyrene](/ProductImageEN1/2025-04/Small/a9783f11-3b5e-46a2-8ebd-cf5ec0bab5ee.gif)
- 2025-12-23
- CAS:50-32-8
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Benzo[a]pyrene
-
![50-32-8 Benzo[a]pyrene](/ProductImageEN2/2025-11/Small/934f077f-6efa-4854-a3d5-fdd58fe1dd6a.png)
- $35.00 / 10mg
- 2025-12-22
- CAS:50-32-8
- Min. Order:
- Purity: 98.70%
- Supply Ability: 10g
- Benzo[A]Pyrene
-
![50-32-8 Benzo[A]Pyrene](https://img.chemicalbook.com/ProductImageEN/2020-1/Small/0a017739-ac7f-48da-af5d-6c36cb6cc4b3.jpg)
- $1.00 / 1KG
- 2025-12-12
- CAS:50-32-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 200000KG