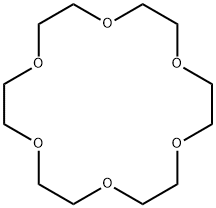
18-Crown-6: Applications in Coordinated Metal Halides and Effect on Oxytocin Stability
Oct 25,2024
General Description
18-Crown-6 plays a crucial role in both the enhancement of luminescent properties and nonlinear optical effects in metal halide coordination compounds, including ZnX2(18-Crown-6) and Mn-based complexes. It demonstrates remarkable quantum yields and unique optical properties, influencing electronic interactions within these complexes. Besides, 18-Crown-6 significantly impacts the stability of oxytocin in aqueous buffers, acting as a stabilizer or destabilizer depending on the buffer composition and pH.

Figure 1. 18-Crown-6
Applications in Coordinated Metal Halides1
Crown ethers, discovered by Pedersen, are known as unique ligands in regard of many aspects. Today, they are available with different ring-opening diameters (e.g., 120−150 pm for 12-crown-4, 450−500 pm for 24-crown-8), and they can contain different heteroatoms such as oxygen, sulfur, or nitrogen as coordinating sites. In inorganic and metal−organic chemistry, crown ethers are known to coordinate almost all types of metal cations. Because of their strong chelating effect and adaptable ring-openings, even alkali metal cations can be strongly bound. Moreover, fascinating compounds were realized with crown ethers as ligands, including, for instance, alkali metal alkalides and electrides, phase-transfer reagents, or low-coordinated nitrogen complexes. 18-Crown-6 is perhaps the most widely applied crown ether. Because of its ring-opening diameter (∼300 pm), 18-crown-6 is especially known for optimal coordination of K + (r:138 pm).
In one study by Claus Feldmann's team, the crown-ether coordination compounds ZnX2 (18-crown-6) and EuX2(18-crown-6) (X: Cl, Br, I) as well as Mn 3Cl6 (18-crown-6) 2, Mn 3I 6(18-crown-6) 2, MnI 2(18-crown-6), and Mn 2I 4(18-crown-6) were prepared by ionic-liquid-based synthesis. The structural features of the title compounds are primarily influenced by the size of the cation in relation to the ring-opening of 18-crown-6 (about 300 pm). In this regard, Mn2+ (r: 83 pm) is neither small enough to clearly prefer off-center (3 + 3) coordination (like Zn 2+ , r: 74 pm) nor large enough for central coordination (like Eu2+ , r: 117 pm).
As a result, Mn3Cl 6(18-crown-6) 2, Mn3 I6 (18-crown-6) 2, and Mn 2I 4(18-crown-6) exhibit unusual single MnX4 tetrahedra coordinated to the crown-ether complex. The structural features of the crown-ether coordination compounds also have major impact on the optical properties. First of all, unexpected emission of all Zn 2+ -containing compounds was observed and could be attributed by computation to charge-transfer transition between the halide np orbital and the zinc 4s orbital. Besides the surprising PL as such, ZnI2 (18-crown-6) shows a remarkable quantum yield of 54%, which is the highest value observed for Zn2+ -based PL. The Mn 2+ - and Eu 2+ -containing crown-ether coordination compounds show bright PL with the characteristic d−d (Mn2+ ) and f−d transitions (Eu 2+ ). Surprisingly, the PL efficiency of Mn 3Cl 6(18-crown-6) 2, Mn3I 6 (18-crown-6)2 , and Mn 2I 4(18-crown-6) even outperform long-optimized commercial phosphors with unprecedented quantum yields of 82, 98, and 100% at ambient temperature. This excellent PL performance can be directly correlated to the structural features of the respective compounds. Besides bright PL, Mn 2I 4 (18-crown-6) shows an anisotropic angle-dependent emission under polarized light and a second-order nonlinear optical effect, which can be again related to its structural features with finite, noninversion symmetric sensitizer−activator Mn 2+−Mn 2+ couples and the presence of a polar, chiral space-group symmetry (P2 12 1 2 1). Such optical properties with bright emission, quantum yields near unity, and NLO effects (including polarized emission, SHG, and visible emission via SHG-driven excitation) are surprising and observed for the first time.
Effect on Oxytocin Stability2
Oxytocin is a nonapeptidic hormone, often used as a drug to combat postpartum hemorrhage, the primary cause of maternal deaths around the globe. Oxytocin also has other numerous biological and psychological functions including a role in lactation and relationships. Additionally, efforts have been made toward using oxytocin as a drug to treat conditions such as alcoholism, autism, and schizophrenia. Unfortunately, oxytocin, in aqueous solutions, degrades rapidly when kept at temperatures above 30 °C. Methods for enhancing oxytocin’s shelf life would therefore be beneficial.
The study found that while 12-crown-4 and 15-crown-5 do not stabilize oxytocin, 18-crown-6 does have a stabilizing effect in citrate/phosphate buffer at pH 4.5. However, in acetate buffer at the same pH, the presence of 18-crown-6 had a destabilizing effect, possibly leading to a different degradation pathway. Both the stabilizing and destabilizing effects, depending on the buffer used, are concentration dependent where a higher concentration of 18-crown-6 is linked to a stronger effect. It is hypothesized that this effect may be linked to 18-crown-6 binding to the protonated ammonium group of oxytocin. Ultraviolet and nuclear magnetic resonance spectroscopy experiments also showed that the presence of 18-crown-6 has an observable effect on the resulting oxytocin spectra.
References:
[1] ELENA MERZLYAKOVA. 18-Crown-6 Coordinated Metal Halides with Bright Luminescence and Nonlinear Optical Effects[J]. Journal of the American Chemical Society, 2021, 143 2: 525-1248. DOI:10.1021/jacs.0c09454.
[2] MOSTAFA GHASEMISARABBADIEH B R S Sveinbjorn Gizurarson. Effect of 18-Crown-6 on Oxytocin Stability in Aqueous Buffer Solutions[J]. ACS Omega, 2021, 6 8: 5075-6030. DOI:10.1021/acsomega.0c06248.
- Related articles
- Related Qustion
- The Synthesis method and Toxicity of 18-Crown-6 May 10, 2024
The compound known as 18-crown-6 is one of the simplest and most valuable of the macrocyclic polyethers.
- 18-Crown-6 coordination compounds: synthesis and properties Jul 3, 2023
18-Crown-6 coordination compounds display intriguing properties in terms of photoluminescence and nonlinear optics.
- 18-Crown-6 - Reaction / Application on Synthetic Works Nov 20, 2019
18-crown-6 (18C6, 18c6, [18]-Crown-6) functions as a ligand for some metal cations, it can solubilize metal salts, particularly potassium salts, in nonpolar and dipolar aprotic solvents. Thus, it is widely used as a phase transfer catalyst.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
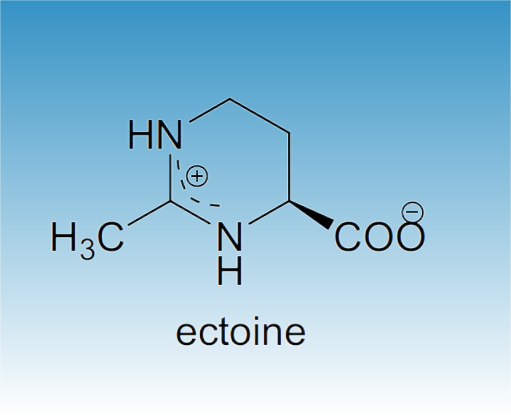
Nov 4,2025Biochemical EngineeringEctoine is an important natural secondary metabolite in halophilic microorganisms. It protects cells against environmental stressors, such as salinity, freezing, drying, and high temperatures.....
Jun 14,2024Drugs18-Crown-6
17455-13-9You may like
- 18-Crown-6
-

- 2025-11-14
- CAS:17455-13-9
- Min. Order:
- Purity: 0.99
- Supply Ability:
- 18-crown - 6 ether
-

- $0.00 / 1PCS
- 2025-11-14
- CAS:17455-13-9
- Min. Order: 1PCS
- Purity: 99%
- Supply Ability: 10 mt
- 18-Crown-6
-

- $0.00 / 1KG
- 2025-11-14
- CAS:17455-13-9
- Min. Order: 1KG
- Purity: 99%min
- Supply Ability: 30tons/month