Diphenylphosphine: Applications in Organometallic Chemistry and Water Treatment
Jul 3,2024
General Description
Diphenylphosphine, a versatile phosphorus-based ligand, stands out in both organometallic chemistry and water treatment applications. In organometallic chemistry, it plays a crucial role in customizing reaction pathways, as seen in the methoxycarbonylation of ethene, impacting selectivity. In water treatment, Diphenylphosphine is essential in creating hybrid porous polymers with exceptional adsorption capacities for dyes and heavy metals, showcasing durability across cycles. These applications underscore Diphenylphosphine's significance in advancing catalysis and environmental remediation efforts, offering tailored solutions for diverse chemical and water treatment challenges.

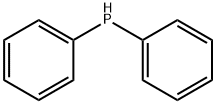
Figure 1. Diphenylphosphine
Applications in Organometallic Chemistry
Influence on Reaction Outcomes
Diphenylphosphine is a phosphorus-based ligand used extensively in the field of organometallic chemistry and catalysis. Its applications, particularly in industrial processes, are pivotal due to its ability to influence reaction mechanisms and outcomes. One such application of diphenylphosphine is in the methoxycarbonylation of ethene, a process significant for producing esters and polymers. In the context of using diphenylphosphine for enhancing selectivity in chemical reactions, its role in the methoxycarbonylation of ethene involves its incorporation into polyhedral oligomeric silsesquioxanes. These are hybrid inorganic-organic compounds where diphenylphosphine groups are attached at the periphery. The inclusion of diphenylphosphine in such macromolecular structures notably affects the reaction outcomes due to the spatial arrangement of the diphenylphosphine moieties and their proximity to the reactive center. The macromolecular architecture significantly influences the performance of diphenylphosphine in these reactions. For instance, variations in the spacer units linking the silicon atoms to the diphenylphosphine groups lead to different reaction products.
Customized Reaction Pathways
Macromolecules with an ethyl spacer between the silicon and the diphenylphosphine moiety, such as G0-8ethylPPh(2) and G1-16ethylPPh(2), specifically yield methyl propanoate. Conversely, a similar structure with a methyl spacer results in the production of a copolymer instead. These observations underline the critical role of diphenylphosphine in determining the selectivity of the methoxycarbonylation of ethene. By altering the length and nature of the spacer between the silicon and diphenylphosphine, chemists can steer the reaction towards producing specific products. This ability to customize the reaction pathway using diphenylphosphine highlights its utility and versatility as a ligand in catalytic processes, making it a valuable tool in the development of advanced materials and chemicals. 1
Applications in Water Treatment
Characteristics of Hybrid Porous Polymers
Diphenylphosphine plays a pivotal role in the development of hybrid porous polymers tailored for effective water treatment applications. These polymers, namely DPPF-HPP and DPPOF-HPP, are synthesized utilizing a Friedel-Crafts reaction involving octavinylsilsesquioxane and either Diphenylphosphine or its oxide derivative (DPPOF). The resultant materials boast exceptional characteristics crucial for water treatment purposes. Both Diphenylphosphine-HPP and DPPOF-HPP showcase substantial surface areas, with values around 890 m2 g-1 and 780 m2 g-1 respectively, coupled with well-defined pore structures comprising micropores and mesopores. These features are instrumental in facilitating their outstanding adsorption capacities. Diphenylphosphine-HPP, for example, impresses with its remarkable adsorption capabilities, reaching 2280 mg g-1 for Congo Red and 1440 mg g-1 for Crystal Violet, underscoring its effectiveness in eliminating various dyes from aqueous solutions. Furthermore, Diphenylphosphine-HPP exhibits a notable affinity for heavy metal ions like Hg2+, demonstrating an adsorption capacity of 300 mg g-1. This characteristic highlights its potential in combatting water pollution issues stemming from heavy metal contamination, thereby bolstering its role in wastewater treatment applications. Crucially, the longevity of these hybrid polymers is emphasized by their durability throughout multiple adsorption-desorption cycles without deterioration.
Efficiency in Water Treatment
This resilience guarantees sustained functionality and dependability in water treatment scenarios, ensuring prolonged efficiency in endeavors aimed at environmental restoration. In essence, Diphenylphosphine-incorporated ferrocene-based hybrid porous polymers signify a significant breakthrough in the realm of materials science geared towards water treatment. Their synthesis process, structural attributes, high adsorption capacities for dyes and heavy metals, and steadfastness under operational conditions collectively position them as highly effective and eco-friendly adsorbents capable of addressing modern challenges in water purification and environmental conservation. 1
Reference
1. Vautravers NR, Cole-Hamilton DJ. Diphenylphosphine containing macromolecules in the methoxycarbonylation of ethene: the effect of macromolecular architecture on the selectivity of the reaction. Dalton Trans. 2009; (12): 2130-2134.
2. Yang X, Liu H. Diphenylphosphine-Substituted Ferrocene/Silsesquioxane-Based Hybrid Porous Polymers as Highly Efficient Adsorbents for Water Treatment. ACS Appl Mater Interfaces. 2019;11(29):26474-26482.
- Related articles
- Related Qustion
In this paper, the knowledge of stearic acid is popularized, and its chemical structure, production, physical properties and application fields are discussed.....
Jul 3,2024APIAt about 1300°C, it decomposes into BaO and CO2. Its vapour pressure is negligible.....
Jul 3,2024Inorganic saltsDiphenylphosphine
829-85-6You may like
Diphenylphosphine manufacturers
- Diphenylphosphine
-

- $0.00 / 1kg
- 2024-01-04
- CAS:829-85-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Diphenylphosphine
-

- $0.00 / 25KG
- 2023-08-14
- CAS:829-85-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Diphenylphosphine
-

- $0.00 / 1KG
- 2022-12-16
- CAS:829-85-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20 mt