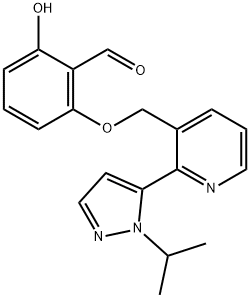
A relaxed-state (R-state) stabilizer of hemoglobin: Voxelotor
Jan 5,2024
Description
Voxelotor, also known as GBT440, is a relaxed-state (R-state) stabilizer of hemoglobin developed by Global Blood Therapeutics and approved by the USFDA for treating sickle cell disease. Voxelotor also has an Orphan Drug designation and Priority Medicine status in Europe for treating sickle cell disease[1].
Use and mechanism of action
Sickle cell disease is a genetic disorder characterized by the mutation of a single amino acid (glutamic acid 6 to valine) in the hemoglobin β-chain. This simple mutation results in hemoglobin polymerization under low-oxygen conditions, responsible for the dysfunctional red blood cell phenotype associated with sickle cell disease. Voxelotor forms a Schiff base with the N-terminal valine of the hemoglobin α-chain, which increases the affinity of sickle cell hemoglobin for oxygen and prevents polymerization. It is an oral agent that modifies the affinity between hemoglobin and oxygen. It acts through a covalent, reversible bond that results in allosteric modification of hemoglobin, resulting in a higher affinity for oxygen[2]. As a result, at the same given Po2, hemoglobin molecules may retain a higher oxygen saturation.
Synthesis method
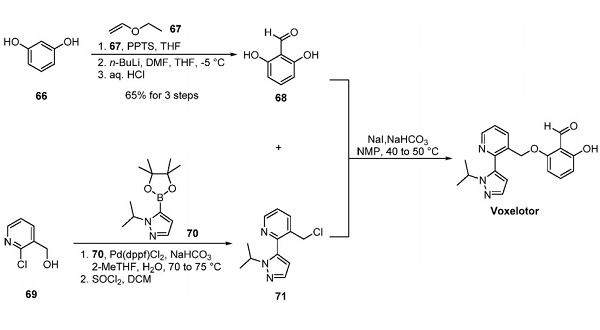
A concise, convergent approach to constructing a voxelotor is described above[3]. Resorcinol (66) was protected as the corresponding bis(ethoxyethyl ether) by treatment with ethyl vinyl ether (67) and pyridinium p-tosylate (PPTS) in THF. The resulting arene underwent directed ortho metalation with n-butyllithum, followed by a DMF quench to install the aldehyde. Removal of the ethoxyethyl groups with aqueous HCl furnished benzaldehyde 68. Separately, (2-chloropyridin- 3-yl)methanol (69) underwent Suzuki coupling with pyrazoloboronate 70, and subsequent treatment with thionyl chloride in dichloromethane provided pyridinyl chloride 71. Finally, the oalkylation of 68 with 71 mediated by sodium iodide and sodium bicarbonate in warm NMP delivered voxelotor.
References
[1] Blair, Hannah A. “Voxelotor: First Approval.” Drugs 80 2 (2020): 209–215.
[2] Omar Al-Qudsi. “Voxelotor as a Treatment of Persistent Hypoxia in the ICU.” Chest 164 1 (2023): e1–e4.
[3] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
- Related articles
- Related Qustion
Surufatinib is synthesised from dichloropyrimidine and hydroxyindole by substitution reaction. This process requires the participation of Surufatinib Amine.....
Jan 5,2024InhibitorsBromobenzene is the simplest member of the class of bromobenzenes, it has a role as a non-polar solvent. Bromobenzene shows different bromination selectivity in polar solvents containing ionic liquid.....
Jan 5,2024Organic ChemistryVoxelotor
1446321-46-5You may like
- Voxelotor
-

- $0.00 / 1kg
- 2024-04-02
- CAS:1446321-46-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20tons
- Voxelotor
-
- $35.00 / 1kg
- 2024-03-29
- CAS:1446321-46-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Voxelotor
-

- $6.68 / 1KG
- 2020-01-09
- CAS: 1446321-46-5
- Min. Order: 1KG
- Purity: 97%-99%
- Supply Ability: 1kg-1000kg