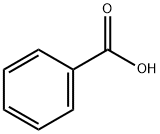
Application and Pharmacology of Benzoic acid
Mar 23,2022
General description
Benzoic acid appears as a white crystalline solid. Slightly soluble in water. The primary hazard is the potential for environmental damage if released. Immediate steps should be taken to limit spread to the environment. Used to make other chemicals, as a food preservative, and for other uses. Benzoic acid is a Nitrogen Binding Agent. The mechanism of action of benzoic acid is as an Ammonium Ion Binding Activit. Benzoic acid is an aromatic carboxylic acid naturally present in plant and animal tissues which can also be produced by microorganisms. Benzoic acid and a wide range of derivatives and related benzenic compounds, such as salts, alkyl esters, parabens, benzyl alcohol, benzaldehyde and benzoyl peroxide, are commonly used as antibacterial and antifungal preservatives and as flavouring agents in food, cosmetic, hygiene, and pharmaceutical products. As the result of their widespread occurrence, production and uses, these compounds are largely distributed in the environment being found in water, soil, and air[1].
Application and Pharmacology
Benzoic acid (C7H6O2) is the simplest aromatic carboxylic acid, with a carboxylic group directly bonded to the benzene ring. It is naturally present in plant and animal tissues, and can also be generated in fermented products through microbial metabolism. It is industrially synthesized and included as preservative and/or flavouring agent in food, cosmetic, hygiene and pharmaceutical products. It is also used as additive, nucleating agent, intermediate, stabilizer and/or catalyst in coolant, solvent, photography, plastic, textiles, pesticide, paper, and dye industries. Benzoic acid is a compound comprising a benzene ring core carrying a carboxylic acid substituent. It has a role as an antimicrobial food preservative, an EC 3.1.1.3 (triacylglycerol lipase) inhibitor, an EC 1.13.11.33 (arachidonate 15-lipoxygenase) inhibitor, a plant metabolite, a human xenobiotic metabolite, an algal metabolite and a drug allergen. It is a conjugate acid of a benzoate. Some benzoic acid derivatives have active bacteriostatic and fragrant properties, and they are used in the pharmaceutical and perfume industry. These molecules are biologically active and often used as building blocks for drugs or other biologically active molecules, with applications ranging from antibacterial substances to UV protective agents. Derivatives of p‐aminobenzoic acid (PABA) have many interesting pharmacological and biological properties, such as AChEIs in the palliative treatment of AD,antimicrobial activity against Grampositive bacteria,[18]and inhibitory properties against novel antibacterial, antifungal, and antiviral targets. Furthermore, enaminones undergo significant chemical reactions to construct valuabl heterocycles and pharmaceutically essential compounds.
Benzoic acid is a small aromatic acid tracer from biomass burning, which has been found in smoke particles from residential wood combustion and from wildfires. Being the atmospheric smoke particles nucleus of condensation that participates in the formation of atmospheric water droplets and of clouds, benzoic acid may easily be present in atmospheric waters due to its high solubility in water. Benzoic acid has been found in cloud water, and rainwater, where it can participate in chemical and photochemical reactions, affecting the atmospheric chemistry, and the climate, through possible changes in the Earth’s albedo. Therefore, it becomes important to evaluate the reactivity and persistency of benzoic acid in atmospheric waters, in addition to other organic compounds from biomass burning, due to the overwhelming number of forest fires that have occurred in the last few years all over the world. In fact, some recent studies have evaluated the reactivity and lifetime of other tracers from biomass burning at the air-water interface.relevant for atmospheric chemistry, e.g. concentrations and pH), namely of phenol and its derivatives, and highlighted that the oxidation of such compounds transforms the initial chemical composition and that may originate light-absorbing compounds[1].
According to the European Commission (2005), benzoic acid and its salts are considered with benzyl alcohol, benzyl acetate and benzaldehyde as a single category group from the human health point of view by the Joint FAO/WHO Expert Committee on Food Additives (JECFA), as they are rapidly metabolized and excreted in urine within 24 hours. These substances are absorbed from the gastrointestinal tract, reaching their maximum peak concentration in plasma within 1-2 hours, and they can be also partially absorbed (22-89%) by dermal route and inhalation (FAO/WHO, 2000). Benzyl alcohol is degraded by oxidation to benzaldehyde in liver and kidneys, mediated by alcohol dehydrogenases and cytochrome P450[2].
Mechanism of action
More than 90% of commercial benzoic acid goes to phenol production, by oxidative decarboxylation, and then to caprolactam and nylon production, by hydrogenation, oximation and Beckmann rearrangement. Derivatives of benzoic acid and related benzenic compounds, such as sodium, potassium and calcium benzoates, alkyl benzoate esters, hydroxybenzoate esters (parabens), benzyl alcohol, benzaldehyde and benzoyl peroxide, can be naturally found and/or chemically synthesized, and are widely used in different industrial sectors. However, adverse reactions, potential toxicological effects and public health concerns have been reported and discussed despite being considered as harmless substances when used under legal limits or Good Manufacturing Practices (GMP)[3].
Synthesis
Benzoic acid and derivatives possess a wide range of chemical reactions, for instance, pericyclic reactions, Michael addition, C–H functionalization/substitution, and so forth, due to the presence of electrophilic and nucleophilic centers.These compounds are common structural motifs in many bioactive natural products and have significant biological activities, such as anti‐inflammatory, antitumor, anticonvulsive, and antibiotic. Further more, benzoic acid derivatives are among versatile hCAIs. They are capable of interacting with the hCAs through a variety of inhibition mechanisms, such as coordination with the metal ion, likely as carboxylate anions, anchoring to the Zn‐bound H2O/OH ion, and occluding the entrance of the hCAs' active site cavity. Heterocyclic molecules are one of the most important compounds in drug discovery. They are found in the majority of drugs as synthetic or natural products. Moreover, heterocyclic derivatives containing an oxygen ornitrogen atom have shown interesting biological activities, and they represent important classes of natural and non‐natural products. The isoquinoline core in this group is an important heterocyclic moiety that is found in a variety of natural products and pharmaceuticals. The iso quinoline alkaloids are a large family of naturally occurring alkaloids with a wide variety of biological activities, including anti‐inflammatory, anti-microbial, antileukemic, and antitumor propertie[3].
Figure1 A general synthetic procedure of Benzoic acid for the target derivatives
Safety
Benzoic acid and its derivatives are widely used as antibacterial and antifungal preservatives in foods, cosmetics, hygiene products, and oral, parenteral and topic medicines . Benzoic acid has even been described to exert activity against picornavirus like bovine enterovirus type 1 and used as veterinary biocidal hygiene product (EU Regulation 528/2012). Their minimum microbicidal concentrations range from 20 to 1200 mg/l at pH 6.0 for different bacterial or fungal species, and their minimum inhibitory concentrations range from 50 to 1000 mg/l. Benzoic acid and its derivatives are also used as flavouring agents in foods, cosmetics, and hygiene products. The toxicology and adverse effects of benzoic acid and its derivatives and their safety levels have always been controversial. Currently, benzoic acid and a great variety of related compounds are generally recognized as safe (GRAS) substances and their use as additives and/or flavouring agents in foods, cosmetics, pharmaceutical and hygiene products, is permitted by FAO/WHO and FDA/USDA. In this context of controversy, the European Food Safety Authority (EFSA) recommended in 2006 the withdrawal of approval for propylparaben (E216) and its sodium salt (E217) after more than 85 years of extended use, while for the rest of parabens the acceptable daily intake (ADI) of 10 mg/kg body weight/day remains and its use in cosmetics is permitted with a maximum concentration for each compound of 0.4% and a total maximum level of 0.8% (FAO/WHO, 2006). More recently, propylparabens have been shown to cause adverse effects such as endocrine disruption, estrogenic activity and reproductive disorders in rats at dietary doses of down to 10 mg/kg body weight/day and these compounds have been related to skin melanomas, testicular and breast cancer.
References
1.Santos P., Cardoso H. B. & Rocha-Santos T. et al., "Oxidation of benzoic acid from biomass burning in atmospheric waters," Environ Pollut, Vol.244(2019), pp.693-704.
2.Del Olmo A., Calzada J. & Nuñez M., "Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy," Critical reviews in food science and nutrition, Vol.57, No.14(2017), pp.3084-3103.
3.Kalaycı M., Türkeş C. & Arslan M. et al., "Novel benzoic acid derivatives: Synthesis and biological evaluation as multitarget acetylcholinesterase and carbonic anhydrase inhibitors," Archiv der Pharmazie, Vol.354, No.3(2021), p.2000282.
References
1.Santos P., Cardoso H. B. & Rocha-Santos T. et al., "Oxidation of benzoic acid from biomass burning in atmospheric waters," Environ Pollut, Vol.244(2019), pp.693-704.
2.Del Olmo A., Calzada J. & Nuñez M., "Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy," Critical reviews in food science and nutrition, Vol.57, No.14(2017), pp.3084-3103.
3.Kalayc? M., Türke? C. & Arslan M. et al., "Novel benzoic acid derivatives: Synthesis and biological evaluation as multitarget acetylcholinesterase and carbonic anhydrase inhibitors," Archiv der Pharmazie, Vol.354, No.3(2021), p.2000282.
- Related articles
- Related Qustion
- Preparation and Synthesis Method of Benzoic Acid Aug 1, 2024
Benzoic acid belongs to the class of organic compounds known as benzoic acids. These are organic Compounds containing a benzene ring which bears at least one carboxyl group.
- Is benzoic acid solubility in water? Mar 5, 2024
Benzoic acid is not very soluble in water. It has a high solubility in hot water while poor solubility in cold water. The solubility of Benzoic acid in water increases when the temperature is increased.
- Is benzoic acid polar or nonpolar? Dec 21, 2023
Benzoic acid is a polar molecule because of a polar functional group (carboxylic acid) which dominates its interactions with other polar substances, such as water, despite having a nonpolar benzene ring as part of its structure.
Pure diethyl malonate (DEM) is a colorless and transparent liquid with the aromatic odor of ether, and the industrial product is a light yellow transparent liquid, which is slightly soluble in water a....
Mar 23,2022Organic Synthesis IntermediateGiven that cefoperazone–sulbactam is the predominant formulation used at the present time, discussion regarding clinical uses will concentrate on this drug combination.....
Mar 23,2022APIBenzoic acid
65-85-0You may like
- Benzoic acid
-

- $3.50 / 1kg
- 2024-11-03
- CAS:65-85-0
- Min. Order: 1kg
- Purity: ≥99%
- Supply Ability: 3000tons/month
- Benzoic acid
-

- $100.00 / 50kg
- 2024-11-03
- CAS:65-85-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000Ton
- Benzoic acid
-

- $0.00 / 25KG
- 2024-11-02
- CAS:65-85-0
- Min. Order: 25KG
- Purity: 98%min
- Supply Ability: 30tons/month