Bimatoprost for the treatment of eyelash, eyebrow and scalp alopecia
Feb 7,2024
General Description
Bimatoprost is a synthetic prostamide F2α analog used to treat ocular hypertension, open-angle glaucoma, and hypotrichosis of the eyelashes. It is administered once daily in the evening to the affected eye(s) and applied directly at the base of the lashes using a specialized applicator for eyelash growth. Bimatoprost binds specifically to prostamide receptors to increase hair growth, length, and thickness by modulating the eyelash growth cycle. The medication exhibits rapid absorption after ocular administration, with a short elimination half-life and low systemic exposure.

Figure 1. Bimatoprost
Overview
Bimatoprost is a synthetic prostamide F2α analog that replicates the ocular effects of the body's natural prostamide. It is utilized primarily for treating ocular hypertension, open-angle glaucoma, and hypotrichosis of the eyelashes. The medication is available in two concentrations, 0.1 mg/mL and 0.3 mg/mL, for reducing elevated intraocular pressure. A single drop is administered once daily in the evening to the affected eye(s). For addressing hypotrichosis of the eyelashes, Bimatoprost is offered as a 0.3 mg/mL solution, which is applied directly at the base of the lashes using a specialized applicator on a nightly basis. Chemically, Bimatoprost is a white to off-white powder with a molecular mass of 415.58. It exhibits high solubility in alcohols, slight solubility in water, and is practically insoluble in toluene and hexane. The ophthalmic solution, branded as LUMIGAN® 0.01% and 0.03%, is isotonic and colorless. Its composition includes bimatoprost, benzalkonium chloride (an antimicrobial preservative), sodium phosphate dibasic heptahydrate, citric acid (buffering agents), sodium chloride (tonicity adjuster), and sodium hydroxide and hydrochloric acid (pH adjusters) in purified water. The pH of the solution ranges from 6.8 to 7.8 throughout its shelf life. 1
Pharmacodynamics
Bimatoprost is a synthetic prostamide F2α analog that belongs to the fatty acid amide family. Structurally and pharmacologically similar to PG F2α 1-ethanolamide (prostamide F2α), it is the first member of the prostamide drug class. Unlike a prodrug, bimatoprost does not require conversion to an active metabolite to exert its pharmacological activity. The pharmacodynamics of bimatoprost involve its specific binding to prostamide receptors, which are distinct from prostanoid FP receptors targeted by PG analogs. Bimatoprost does not interact with adrenergic, cholinergic, cannabinoid, dopaminergic, or prostanoid receptors. In murine models, it has been observed that bimatoprost stimulates hair growth and increases eyelash length without generating new follicles. It affects the eyelash growth cycle by increasing the percentage of anagen follicles, promoting anagen reentry of telogen eyelash follicles, and prolonging the anagen phase while arresting catagen entry. Additionally, bimatoprost increases eyelash thickness by increasing hair bulb diameter. Overall, bimatoprost's pharmacological effects on eyelashes involve specific interactions with prostamide receptors, leading to increased hair growth, length, and thickness through modulation of the eyelash growth cycle. 2
Pharmacokinetics
Following administration of bimatoprost ophthalmic solution, the maximum plasma concentration is achieved within 10 minutes, and the lowest concentration is observed after 1.5 hours. The majority of the drug remains in the plasma and is moderately distributed into body tissues, with a steady state volume of approximately 0.67 liters per kilogram. Bimatoprost exhibits a plasma protein binding of around 88%. The elimination half-life of bimatoprost is approximately 45 minutes. When administered intravenously, approximately two-thirds of the total elimination of bimatoprost occurs through urinary excretion, while the remainder is excreted in feces. However, systemic exposure to bimatoprost is minimal after ocular administration. When bimatoprost ophthalmic solution is applied directly at the base of the eyelashes using an applicator, only about 5% of the volume of the drop administered for glaucoma treatment reaches the target area. In summary, bimatoprost demonstrates rapid absorption after ocular administration, with a short elimination half-life and low systemic exposure. The majority of the drug remains in the plasma, and only a small fraction reaches the base of the eyelashes when applied topically. 3
Reference
1. LATISSE? Prescribing Information – Allergan. [cited 2016 June 20]
2. Barrón-Hernández YL, Tosti A. Bimatoprost for the treatment of eyelash, eyebrow and scalp alopecia. Expert Opin Investig Drugs. 2017;26(4):515-522.
3. FDA Briefing Document Dermatologic and Ophthalmic Drugs Advisory Committee Briefing Document. [cited 2016 June 20].
- Related articles
- Related Qustion
- Bimatoprost: Its bioactivity, mechanism, and pharmacology Mar 20, 2023
Bimatoprost is one compound in a new class of highly efficacious ocular hypotensive agents. It is pharmacologically unique and appears to mimic the prostamides.
- The uses and side effects of Bimatoprost Sep 17, 2019
Bimatoprost, also known as Latisse or Lumigan, belongs to a group of drugs called prostamides, which are synthetic structural analogs of prostaglandin. Bimatoprost, marketed by Allergan, is administered in an ophthalmic solution and has the
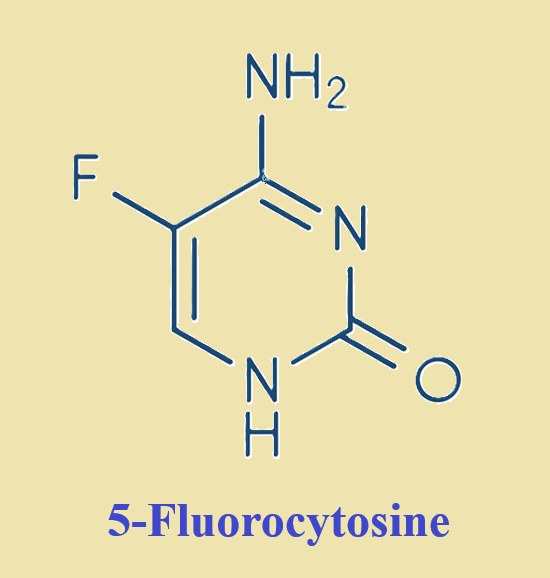
Yes. The therapeutic fluoropyrimidines 5-fluorouracil (5-FU) and 5-fluorocytosine (5-FC) have long been used to treat human cancer and severe invasive fungal infections, respectively.....
Nov 1,2024Biochemical EngineeringExemestane effectively treats hormone-sensitive breast cancer in postmenopausal women, showing superior efficacy and safety in clinical trials compared to megestrol.....
Feb 7,2024APIBimatoprost
155206-00-1You may like
- Bimatoprost
-

- $100.00/ kg
- 2024-11-16
- CAS:155206-00-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000
- Bimatoprost
-

- $30.00 / 2mg
- 2024-11-15
- CAS:155206-00-1
- Min. Order:
- Purity: 99.81%
- Supply Ability: 10g
- Bimatoprost
-

- $0.00 / 1KG
- 2024-11-15
- CAS:155206-00-1
- Min. Order: 0.10000000149011612KG
- Purity: 99.5% up by HPLC
- Supply Ability: 20 tons