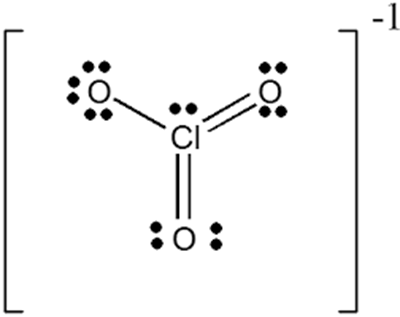
In ClO3-, what is the oxidation state of chlorine?
Feb 27,2024
The oxidation state of chlorine is +5 in ClO3-. The Cl forms double bonds with two of the oxygen molecules and a single bond with the third. This leaves the third oxygen negatively charged with a single unpaired electron. The oxidation state of chlorine in ClO3- is the sum of the oxidation number provided by the anions (-2 for each oxygen), less the balance charge on the compound (-1 for ClO3-). Thus, the chlorine oxidation state is -2x3 - (-1) = +5 (negative charges must equal positive charge).
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringAmoxicillin, a widely used antibiotic for bacterial infections, is essential to understand its shelf life. This article will show the proper method to store amoxicillin and its shelf life.....
Feb 27,2024APIYou may like






