Kaempferol: Chemical Characteristics, Biosynthetic Pathways and Bioavailability
Jun 11,2024
General Description
Kaempferol, a flavonol compound, belongs to the flavonoid family and exhibits antioxidant properties with various health benefits. It is synthesized in plants via the shikimic acid pathway and commonly found in glycoside form, enhancing its solubility. The bioavailability of kaempferol, ingested mainly as glycosides, is influenced by saccharide attachments. Absorption occurs in the small intestine through passive diffusion, with metabolism in the liver and enterocytes. Its metabolites are distributed in body tissues and excreted in urine. Kaempferol's diverse biological activities, including anti-carcinogenic and anti-inflammatory properties, make it a subject of extensive research for potential therapeutic applications.

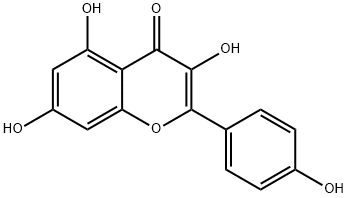
Figure 1. Kaempferol
Chemical Characteristics
Kaempferol, classified as a flavonol, is a prominent member of the flavonoid family, which constitutes the largest group of secondary plant metabolites. Flavonoids, including kaempferol, are polyphenolic compounds characterized by their low molecular weight. These compounds play crucial roles in plant growth stimulation, regulation, and defense mechanisms. Chemically, all flavonoids share a fundamental structure: a 15-carbon benzopyranone or benzopyran, with a three-carbon bridge cyclized with oxygen, forming a C6-C3-C6 flavan nucleus. Kaempferol, with a molecular formula of C15H10O6, adheres to this structure. Kaempferol's significance extends beyond its chemical composition. It is renowned for its antioxidant properties, which contribute to various health benefits. Research has identified over 104 types of flavonoids, highlighting their diverse pharmacological effects, including hepatoprotective, antimicrobial, renoprotective, antidiabetic, cardioprotective, anti-arthritic, neuroprotective, gastroprotective, and anti-mutagenic properties. Recent studies have intensified focus on kaempferol's potential anti-carcinogenic properties, supported by evidence linking its consumption to reduced cancer incidence. Furthermore, its anti-inflammatory and anti-adipogenic potentials have garnered attention, expanding its therapeutic implications. In summary, kaempferol, as a flavonol, embodies the chemical characteristics typical of flavonoids while exhibiting diverse biological activities, making it a subject of extensive research and a promising candidate for various therapeutic applications. 1
Biosynthetic Pathways
Kaempferol, a flavonol compound, is synthesized in plants through the shikimic acid pathway, predominantly occurring within plastids. This pathway is responsible for producing a wide array of flavonoids, with over 2000 compounds identified, among which nearly 500 exist in a free-aglycone state, while others are found as O- or C-glycosides. In its free form, kaempferol possesses lipophilic properties. However, within plants, it is commonly found in glycoside form, attached to a sugar moiety, making it water-soluble. The hydroxyl functional groups present in kaempferol serve as potential sites for linkage to saccharides, resulting in O-glycosides. The sugars most frequently attached to kaempferol include monosaccharides such as glucose, rhamnose, galactose, arabinose, and xylose, as well as the disaccharide rutinose, composed of glucose and rhamnose connected by a β-glycosidic bond. This glycosylation enhances the solubility and stability of kaempferol within the plant system, facilitating its various physiological functions. 2
Bioavailability
Kaempferol, a flavonol commonly ingested as glycosides, has been the subject of extensive pharmacokinetic studies in both in vitro and in vivo settings involving rats and humans. The bioavailability and bioactivity of kaempferol are significantly impacted by the types and attachments of saccharides within the glycosides. The highly polar nature of glycosides affects their absorption, while the intermediate polarity of aglycones facilitates it. Some glycosides require prior hydrolysis to be absorbed, whereas others can be absorbed without this process. In the small intestine, kaempferol is primarily absorbed due to the lipophilicity of its aglycone form, which enables passive diffusion. However, evidence suggests that facilitated diffusion or active transport mechanisms may also play a role in its absorption. The nature of sugar linking influences compound uptake, with enterocytes showing a preference for glucose and membrane-bound beta-glucosidase breaking down glucosides before absorption. Following absorption, kaempferol and its metabolites can reach systemic circulation and tissues, where they undergo metabolism in the liver and enterocytes via phase I (oxidation and O-demethylation) and phase II pathways (sulfation, glucuronidation, and methylation). Subsequently, these metabolites are distributed to body tissues and excreted in urine. Pure kaempferol can be isolated from various plant species, and its glycosides are identified in many plant families. The detailed account of kaempferol chemical compounds and derivatives can be found in recent studies. Additionally, the kaempferol content of common foodstuffs is provided in relevant literature. Overall, understanding the bioavailability of kaempferol is crucial for assessing its potential health benefits and designing effective therapeutic interventions. 2
Reference
1. Dabeek WM, Marra MV. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients. 2019;11(10):2288. Published 2019 Sep 25. doi:10.3390/nu11102288
2. Periferakis A, Periferakis K, Badarau IA, et al. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int J Mol Sci. 2022;23(23):15054.
- Related articles
- Related Qustion
- What is Kaempferol? Feb 12, 2020
Kaempferol (3,5,7‐trihydroxy‐2‐[4‐hydroxyphenyl]‐4H‐1‐benzopyran‐4‐one) is a yellow bioactive flavonoid, which is present inmany edible plants such as tea, cabbage, broccoli, endive, kale, beans, tomato, strawberries, leek, and grapes.
1,7-Dimethylxanthine is a naturally occurring alkaloid compound that can enhance alertness and reduce drowsiness.....
Feb 27,2025APIMagnesium hydroxide is a layer-structured mineral, and there are a family of double hydroxides where the magnesium hydroxide layers alternate with trivalent metal hydroxide layers.....
Jun 11,2024APIKaempferol
520-18-3You may like
- Kaempferol
-
- 2025-10-31
- CAS:520-18-3
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Kaempferol
-

- $58.00 / 100mg
- 2025-10-31
- CAS:520-18-3
- Min. Order:
- Purity: 99.09%
- Supply Ability: 10g
- Kaempferol
-

- $0.00 / 1kg
- 2025-10-31
- CAS:520-18-3
- Min. Order: 1kg
- Purity: 98%min USP
- Supply Ability: 100kg