Lambrolizumab: Mechanism, Biological Activities and Adverse effects
Mar 21,2023
General Description
Lambrolizumab, also known as Pembrolizumab, sold under the name Keytruda, is a PD-1 blocking antibody used to treat various types of cancer, including metastatic melanoma, non small-cell lung cancer, cervical cancer, head and neck cancer, and Hodgkin's lymphoma. Lambrolizumab is a highly selective IgG4-kappa humanized monoclonal antibody against PD-1 receptors. It was generated by grafting the variable sequences of a very high-affinity mouse antihuman PD-1 antibody onto a human IgG4-kappa isotype containing a stabilizing S228P Fc mutation. It contains 32 cysteine residues and the complete folded molecule includes 4 disulfide linkages as interchain bonds and 23 interchain bonds. It was developed by Merck & Co and first approved for the treatment of metastatic malignant melanoma by the FDA on September 4, 2014,becoming the first approved therapy against PD-1. In the time since its initial approval, Lambrolizumab has been granted approval in the treatment of a wide variety of cancers.1-2
Figure 1. Properties of Lambrolizumab Injection
Mechanism of Action
Lambrolizumab binds with high affinity to the cell surface receptor programmed cell death protein 1 (PD-1) and antagonizes its interaction with its known ligands PD-L1 and PD-L2. Under normal circumstances, the binding of the ligands of PD-1 to the receptor inhibits the TCR-mediated T-cell proliferation and cytokine production. This inhibitory signal appears to play a role in self-tolerance and collateral damage minimization after immune responses against a pathogen and maternal tolerance to fetal tissue. The binding of pembrolizumab to PD-1 prevents this inhibitory pathway, causing a physiological shift towards immune reactivity and enhancing tumor immunosurveillance and anti-tumor immune response.3
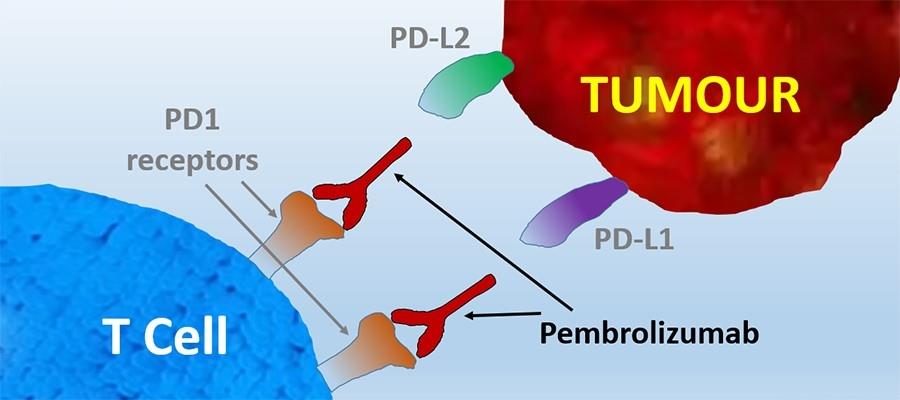
Figure 2. Mechanism of Action of Lambrolizumaba
Uses for Lambrolizumab
Melanoma
1. for the treatment of patients with unresectable or metastatic melanoma (adult patients in the US8 and patients ≥12 years old in the EU.
2. for the adjuvant treatment of adult and pediatric patients 12 years of age and older with Stage IIB, IIC, or III melanoma following complete resection.
Non-Small Cell Lung Cancer (NSCLC)
1. in combination with pemetrexed and platinum-based chemotherapy as a first-line treatment for patients with metastatic nonsquamous NSCLC with no EGFR or ALK mutations.
2. in combination with carboplatin and paclitaxel as a first-line treatment for patients with metastatic squamous NSCLC.
3. as a monotherapy for the first-line treatment of NSCLC expressing PD-L1 with no EGFR or ALK mutations in patients with metastatic disease or stage III disease who are not candidates for surgery or chemoradiation.
4. as a monotherapy for the treatment of NSCLC expressing PD-L1 with disease progression on or after platinum-based chemotherapy - this includes patients with EGFR or ALK mutations, providing they have experienced disease progression on prior FDA-approved therapy for these aberrations.
Head and Neck Squamous Cell Cancer (HNSCC)
1. in combination with fluorouracil and platinum-based chemotherapy as a first-line treatment for patients with metastatic or recurrent, unresectable HNSCC.
2. as a monotherapy for the first-line treatment of patients with metastatic or recurrent, unresectable HNSCC expressing PD-L1.
3. as a monotherapy for the treatment of patients with metastatic or recurrent HNSCC with disease progression on or after platinum-based chemotherapy.
Cervical Cancer
1. in combination with other chemotherapies, with or without bevacizumab, for the treatment of persistent, recurrent, or metastatic cervical cancer expressing PD-L1.
2. as a monotherapy for the treatment of recurrent or metastatic cervical cancer expressing PD-L1 in patients who have experienced disease progression on or after previous chemotherapy.
Classical Hodgkin Lymphoma (cHL)
1. for the treatment of adult patients with relapsed or refractory cHL.
2. for the treatment of pediatric patients with refractory cHL, or cHL that has relapsed following ≥2 lines of therapy.
Triple-Negative Breast Cancer (TNBC)
1. for the treatment of patients with high-risk early-stage TNBC, in combination with chemotherapy as a neoadjuvant treatment followed by continued use as a single adjuvant agent following surgery.
2. in combination with chemotherapy for the treatment of locally recurrent unresectable or metastatic TNBC expressing PD-L1.
For all approved adult indications, Lambrolizumab may be used for an additional 6 weeks at 400mg weekly.4
Pharmacokinetics
Absorption
AUC, peak plasma concentration, and trough concentration of Lambrolizumab are dose proportional over the dosage range of 2–10 mg/kg every 3 weeks; systemic accumulation is 2.1-fold when administered every 3 weeks. Based on dose-exposure efficacy and safety relationships, no clinically important differences between 200 mg every 3 weeks and 2 mg/kg every 3 weeks in patients with melanoma or NSCLC.5
Elimination
The half life of Lambrolizumab is 22 days. Clearance is approximately 23% lower at steady state than following initial dose; difference not clinically important.5
Metabolism
Lambrolizumab is catalyzed into smaller peptides and amino acids via general protein degradation.5
Pharmacodynamics
Lambrolizumab exerts its pharmacologic effects by releasing PD-1 pathway-mediated inhibition of the immune response, which in turn improves the anti-tumor immune response. Due to its relatively broad mechanism of action, it is useful in the treatment of a wide variety of cancers. Lambrolizumab can cause immune-mediated adverse reactions - including hepatitis, nephritis, and pneumonitis - in any organ system or tissue. Careful monitoring of the patient (including laboratory evaluation of liver, kidney, and thyroid function) should occur at baseline and periodically throughout therapy to monitor for emerging immune-mediated reactions.6
Adverse effects
People have had severe infusion-related reactions to Lambrolizumab. The common adverse reactions have been fatigue (24%), rash (19%), itchiness (pruritus) (17%), diarrhea (12%), nausea (11%) and joint pain (arthralgia) (10%). Other adverse effects occurring in between 1% and 10% of people taking Lambrolizumab have included anemia, decreased appetite, headache, dizziness, distortion of the sense of taste, dry eye, high blood pressure, abdominal pain, constipation, dry mouth, severe skin reactions, vitiligo, various kinds of acne, dry skin, eczema, muscle pain, pain in a limb, arthritis, weakness, edema, fever, chills, myasthenia gravis, and flu-like symptoms.7
Chemistry and manufacturing
Pembrolizumab is an immunoglobulin G4, with a variable region against the human PD-1 receptor, a humanized mouse monoclonal [228-L-proline(H10-S>P)]γ4 heavy chain (134-218') disulfide and a humanized mouse monoclonal κ light chain dimer (226-226:229-229)-bisdisulfide. It is recombinantly manufactured in Chinese hamster ovary (CHO) cells.8
References
1. Robert C; et al. Lancet. 2014 Sep 20;384(9948):1109-17.
2. Poole RM; et al. Drugs. 2014 Oct;74(16):1973-81.
3. FDA Approved Drug Products: Keytruda (pembrolizumab) solution for intravenous injection.
4. Khoja L; et al. J Immunother Cancer. 2015 Aug 18;3:36.
5. Jazirehi AR; et al. Am J Cancer Res. 2016 Oct 1;6(10):2117-2128.
6. Health Canada Approved Drug Products: KEYTRUDA (pembrolizumab) solution for intravenous infusion.
7. Keytruda 50 mg powder for concentrate for solution for infusion - Summary of Product Characteristics (SmPC)". (emc). 25 November 2019.
8. "Keytruda EPAR". European Medicines Agency (EMA). Archived from the original on 24 May 2020.
- Related articles
- Related Qustion
- Unveiling Lambrolizumab: A Comprehensive Insight into Its Production, Mechanism, and Clinical Implications Apr 11, 2024
Lambrolizumab,represents a groundbreaking advancement in the realm of immunotherapy drugs, primarily employed in the battle against various types of cancer.
- Lambrolizumab: Mechanism, Bioactivity and Toxiological Studies Jan 4, 2023
Pembrolizumabis a PD-1 inhibitor that has shown clinical activity in a variety of solid tumors.
3'-Methoxypropiophenone (3-MOP) is an organic compound belonging to the ketone class of compounds.....
Mar 21,2023APIL-Ergothioneine (EGT) is a L-histidine derivative that is N(alpha), N(alpha),N(alpha)-trimethyl-L-histidine in which the hydrogen at position 2 on the imdazole ring is replaced by a mercapto group....
Mar 23,2023Food AdditivesPembrolizumab
1374853-91-4You may like
- What is the nitration process of Methyl benzoate?
Nov 2, 2024
- What are the Benefits of Tributyrin?
Nov 2, 2024
- What is Anisole used as?
Nov 2, 2024
Pembrolizumab manufacturers
- Lambrolizumab
-

- $0.00 / 500MG
- 2024-11-02
- CAS:1374853-91-4
- Min. Order: 500MG
- Purity: 98%min
- Supply Ability: 30KG/month
- Pembrolizumab
-

- $218.00 / 1mg
- 2024-10-31
- CAS:1374853-91-4
- Min. Order:
- Purity: 99.70%
- Supply Ability: 10g
- Lambrolizumab
-

- $0.00 / 10g
- 2024-09-25
- CAS:1374853-91-4
- Min. Order: 10g
- Purity: 99%
- Supply Ability: 50kg