Synthesis and Applications of 1,2-Benzisothiazol-3(2H)-one in Bioorganic Chemistry
May 28,2024
General Description
1,2-Benzisothiazol-3(2H)-one is a crucial compound in bioorganic chemistry, primarily utilized in developing potent inhibitors for enzymes like caspase-3 involved in apoptosis regulation. This versatile molecule and its derivatives show promising therapeutic potential in conditions marked by heightened apoptosis, such as neurodegenerative disorders and cancer. The synthesis of 1,2-Benzisothiazol-3(2H)-one involves precise steps, leading to a high-purity product crucial for its organic reactivity. Molecular studies guide structural modifications enhancing interactions with caspase-3, improving inhibition efficacy. Continuous advancements underscore the significance of 1,2-Benzisothiazol-3(2H)-one derivatives as novel caspase inhibitors, offering a novel approach to combat dysregulated apoptosis in various diseases.

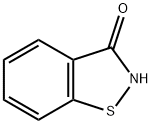
Figure 1. 1,2-Benzisothiazol-3(2H)-one
Bioorganic Chemistry Applications
1,2-Benzisothiazol-3(2H)-one is a versatile compound in bioorganic chemistry, particularly in the development of inhibitors for enzymes like caspase-3, which play critical roles in apoptosis, or programmed cell death. The relevance of 1,2-Benzisothiazol-3(2H)-one extends to therapeutic applications, especially in conditions characterized by excessive apoptosis, such as neurodegenerative diseases, cancer, and autoimmune disorders. Recent advancements in the field highlight the synthesis and evaluation of derivatives of 1,2-Benzisothiazol-3(2H)-one as potent inhibitors of caspase-3. These derivatives have been engineered through structural modifications from the backbone of the original 1,2-Benzisothiazol-3(2H)-one molecule, identified through high-throughput screening processes. The modifications are designed to enhance the molecular interactions with caspase-3, thus increasing the efficacy of inhibition. The effectiveness of these 1,2-Benzisothiazol-3(2H)-one derivatives has been quantified in several studies, with some compounds showing inhibitory concentrations (IC50) in the nanomolar range. This potency underscores the potential of 1,2-Benzisothiazol-3(2H)-one derivatives in medical applications, offering a new approach to controlling apoptosis in pathological conditions. Molecular modeling studies have played a crucial role in understanding how 1,2-Benzisothiazol-3(2H)-one derivatives interact with caspase-3. These studies provide insights into the binding affinities and structural conformations that are most effective for inhibiting the caspase-3 enzyme. Such insights are invaluable for guiding further modifications to the 1,2-Benzisothiazol-3(2H)-one structure, aiming to optimize its interaction with the enzyme and maximize therapeutic benefits. In summary, 1,2-Benzisothiazol-3(2H)-one serves as a foundational compound for generating novel inhibitors of caspase-3, offering promising directions for therapeutic strategies against diseases where apoptosis is dysregulated. The continuous development of 1,2-Benzisothiazol-3(2H)-one derivatives highlights its significant potential in bioorganic chemistry and medicine, particularly as a candidate for novel, more effective caspase inhibitors. 1
Preparation Method
1,2-Benzisothiazol-3(2H)-one, a compound of significant interest in chemical synthesis, can be prepared through several well-defined steps, ensuring both high yield and purity. This synthesis starts by reacting 2-(alkylthio)benzonitriles with halogenating agents in an aqueous environment. For clarity, 1,2-Benzisothiazol-3(2H)-one serves as a pivotal intermediate and end-product in various organic reactions. The preparation of 1,2-Benzisothiazol-3(2H)-one involves initially introducing chlorine to a solution of 2-(alkylthio)benzonitrile and hydrochloric acid, maintained at temperatures between 45-50°C for approximately 2 hours. This reaction step is crucial as it helps to halogenate the benzonitrile, forming a more reactive species that further undergoes transformation. Following halogenation, the mixture is heated to a temperature range of 65-70°C. This step is designed to advance the chemical reactions towards forming 1,2-Benzisothiazol-3(2H)-one. The reaction mixture's pH is then adjusted to around 9, a critical step that facilitates the formation of the alkali salts of 1,2-Benzisothiazol-3(2H)-one, which are key intermediates in the synthesis pathway. Subsequently, the mixture is treated at 100°C for an additional 2 hours to ensure complete reaction before being acidified. This acidification step is vital as it leads to the precipitation of high-purity 1,2-Benzisothiazol-3(2H)-one. The final product of this method results in 1,2-Benzisothiazol-3(2H)-one with an impressive 99.9% purity and a yield of 99.5%, demonstrating the effectiveness of this synthetic route. This method not only highlights the compound's critical role in organic chemistry but also underscores the precision needed in its synthesis to achieve such high purity and yield. 2
Reference
1. Liu D, Tian Z, Yan Z, et al. Design, synthesis and evaluation of 1,2-benzisothiazol-3-one derivatives as potent caspase-3 inhibitors. Bioorg Med Chem. 2013; 21(11): 2960-2967.
2. Hiyama T, Yamamoto M. Preparation of high-purity 1,2-benzisothiazolin-3-ones. 2013; Patent Number: JP2013043882.
- Related articles
- Related Qustion
- 1,2-Benzisothiazol-3(2H)-one(BIT) Apr 22, 2022
1,2-Benzisothiazol-3(2H)-one, also known as benzisothiazolone or BIT, belongs to the class of organic compounds known as benzothiazoles.
p-Anisaldehyde is a colorless liquid with a strong aroma. It has sweet, floral and strong aniseed odor.....
Nov 13,2024Organic ChemistryHexapeptide-11 has been shown to be effective in improving skin elasticity, enhancing nuclear accumulation of Nrf2, increasing proteasomal peptidase activity, inhibiting cellular senescence and facilitating the conversion of young vellus ha....
May 28,2024API1,2-Benzisothiazol-3(2H)-one
2634-33-5You may like
1,2-Benzisothiazol-3(2H)-one manufacturers
- Benzisothiazolone
-

- $32.00/ kg
- 2024-11-15
- CAS:2634-33-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000kg/week
- 1,2-Benzisothiazolin-3-one
-

- $10.00 / 25KG
- 2024-10-28
- CAS:2634-33-5
- Min. Order: 1KG
- Purity: 99.5%
- Supply Ability: 100
- 1,2-benzisothiazolin-3-one
-

- $12.00 / 1KG
- 2024-10-11
- CAS:2634-33-5
- Min. Order: 1KG
- Purity: 99.8%
- Supply Ability: 5000kg