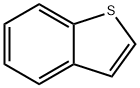
Thianaphthene: A Versatile Chemical Compound
Oct 22,2024
Thianaphthene is an aromatic organic compound with a molecular formula C8H6S and an odor similar to naphthalene (mothballs). It occurs naturally as a constituent of petroleum-related deposits such as lignite tar.
Use
Thianaphthene finds use in research as a starting material for the synthesis of larger, usually bioactive structures. It is found within the chemical structures of pharmaceutical drugs such as raloxifene, zileuton, and sertaconazole, and also BTCP. It is also used in the manufacturing of dyes such as thioindigo.
Thianaphthene may be used in the preparation of 2-thianapthenylphenyllithium, via metalation by n-butyllithium.
Synthesis
Most syntheses of benzothiophene create substituted benzothiophenes as a precursor to further reactions. An example is the reaction of an alkyne-substituted 2-bromobenzene with either sodium sulfide or potassium sulfide to form benzothiophene with an alkyl substitution at position 2.
- Related articles
- Related Qustion
- Synthesis of Benzothiophene Jan 27, 2022
Benzothiophene is constituted by fusion of the benzene ring with the thiophene ring. There are two possible methods of fusion of the benzene ring with two different sites, namely 2,3- or [b] and 3,4- or [c] sites of the thiophene ring and a
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringPromethazine hydrochloride is used to prevent and treat nausea and vomiting related to certain conditions (such as before/after surgery, motion sickness). It is also used to treat allergy symptoms such as rash, itching, and runny nose.....
Oct 22,2024APIThianaphthene
95-15-8You may like
- Thianaphthene
-
- 2025-11-16
- CAS:95-15-8
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Thianaphthene
-

- $10.00 / 1KG
- 2025-11-14
- CAS:95-15-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Thianaphthene
-

- $50.00 / 1KG
- 2025-09-25
- CAS:95-15-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available