Uses of aureobasidin A
Apr 1,2022
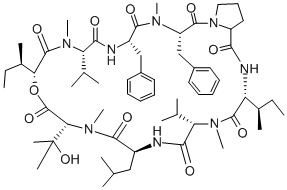
Aureobasidins are a group of 18 natural compounds produced by Aureobasidium pullulans. The prototypic antifungal of the group, aureobasidin A, is a cyclic depsipeptide composed of eight lipophilic amino acids linked through one hydroxyl acid to form the ring structure. Khafrefungin, a natural fungal product produced by an unidentified fungus, was discovered in the course of screening for new antifungal agents at the Merck laboratories.
Analogs of khafrefungin were designed and synthesized but none had increased potency and most had lost antifungal activity. Rustmicin is a macrolide antifungal produced by Micromonospora chalcea and Streptomyces galbus. Although not a drug candidate itself, kahafrefungin has fungicidal activity against the yeasts C. albicans and C. neoformans (but not Aspergillus), selectively inhibits fungal cell IPC, and does not affect the synthesis of mammalian phospholipids. Rustmicin is especially potent against C. neoformans (MICs 0.002 mg/ml or less) and is active against Candida species (MIC r2 mg/ml) with the possible exception of C. guillermondii, but not Aspergillus species.

Uses
The marketability of aureobasidin A, which appears in vivo to show promise only as an anti-Candida agent, but with potential efficacy in histoplasmosis and blastomycosis, is yet to be determined. Based on its unique target, this class of compound warrants further study in azole-resistant candidiasis and endemic mycoses. IPC synthase inhibitors may also be useful in combination therapy. They have the potential to increase the sensitivity of fungi to established antifungal drugs by disrupting the localization and function of the drug efflux pump Cdr1p and hence increasing intracellular concentration of these agents.
Mechanism of action
Sphingolipids are key components of fungal and mammalian membranes, acting as membrane stabilizers through biophysical interactions with phospholipids and sterols. Metabolites of sphingolipid biosynthetic pathways are important second messengers in several biological processes including stress responses, apoptosis, cell cycle regulation, and host immune responses. Aureobasidin A selectively inhibits the biosynthesis of complex fungal sphingolipids at the level of the enzyme IPC synthase, which catalyzes the transfer of phosphoinositol from phosphatidyl inositol to phytoceramide in fungi (ceramide in mammalian cells) to form IPC. Inhibition of sphingolipid synthesis probably reduces fungal growth via the accumulation of phytoceramide, which was shown to induce an apoptosis-like death in S. cerevisiae.
IC50s of 3–5 ng/ml were achieved against IPC synthase in membrane preparations from Candida (MICs o2 mg/ml) and Aspergillus spp. (MICs W25–50 mg/ml), indicating that the resistance of most Aspergillus spp. to aureobasidin is due to another mechanism, most likely increased drug efflux. Khafrefungin and rustmicin also selectively inhibit fungal IPC synthase.
Toxicity
Since aureobasidin selectively inhibits fungal IPC synthase and was well tolerated in mice, toxicity is anticipated to be low.
- Related articles
- Related Qustion
Phloroglucinol is a benzenetriol with hydroxy groups at position 1, 3 and 5. It has a role as an algal metabolite. Phloroglucinol is a natural product found in Artemisia dolosa, Koenigia coriaria, and....
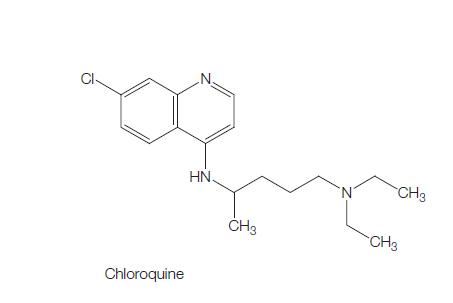
Mar 31,2022DrugsThe molecular formula of chloroquine is C18H26ClN3 and its molecular weight is 320. Chloroquine (Aralen) is formulated as tablets containing 100 or 155 mg of chloroquine base as the hydrochloride, phosphate, or sulfate salt. It is rapidly a....
Apr 1,2022APIBasifungin
127785-64-2You may like
Basifungin manufacturers
- Aureobasidin A
-

- $122.00 / 1mg
- 2024-11-14
- CAS:127785-64-2
- Min. Order:
- Purity: 99.79%
- Supply Ability: 10g