Toxicity of action of Chloroquine
Apr 1,2022
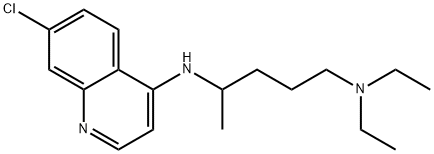
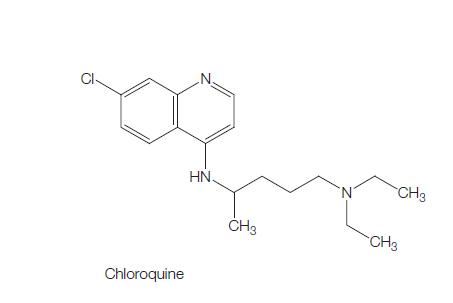
Chloroquine (7-chloro-4[4-diethylamino-1-methlybutylamino]) is a 4- aminoquinoline derivative that has been used extensively for the treatment and prevention of malaria.
The molecular formula of chloroquine is C18H26ClN3 and its molecular weight is 320. Chloroquine (Aralen) is formulated as tablets containing 100 or 155 mg of chloroquine base as the hydrochloride, phosphate, or sulfate salt. It is rapidly absorbed and has a long elimination half-life. Chloroquine’s hydroxyl analog, hydroxychloroquine (Plaquenil), has similar properties and activity, and its lower toxicity favors its use when prolonged treatment is required. The chemical structures of chloroquine, hydroxychloroquine, and the active metabolite desethylchloroquine (DCQ) are shown in Figure 166.1. Chloroquine exerts is antimalarial activity by inhibiting the detoxification of heme within the digestive vacuole of the intraerythrocytic malarial parasite. Through additional antimicrobial mechanisms, chloroquine has activity against a range of other parasites, fungi, bacteria, and viruses.
Mechanism of action
During intraerythrocytic growth and replication of malaria, hemoglobin is utilized as the principal source of nutrition, and its digestion takes place within the parasite digestive vacuole. The digestion of hemoglobin leads to the formation of heme, which is immediately oxidized to ferriprotoporphyrin IX (FPIX). FPIX is highly toxic to cells, leading to increased membrane permeability and cell lysis. The detoxification of FPIX is therefore crucial to parasite survival, and is achieved through the conversion of FPIX to the inactive and insoluble crystalline hemozoin (malaria pigment).
Although the antimalarial activity of chloroquine is incompletely understood, it is believed that chloroquine accumulates within the digestive vacuole and binds with high affinity to FPIX to prevent its detoxification to hemozoin.
In addition to antimalarial activity, the weak basic property of chloroquine and hydroxychloroquine affords them activity against several intracellular bacteria and fungi as well as certain viruses. Chloroquine and hydroxychloroquine accumulate within acidic organelles such as endosomes, lysosomes, and Golgi vesicles; the subsequent alkalinization of these vesicles in cells infected with certain intracellular bacteria and fungi leads to inhibition of the growth of these organisms. This mechanism of action, first demonstrated with C. burnetii, also applies to T. whipplei, with chloroquine restoring the bactericidal effect of doxycycline.
Chloroquine may also inhibit the multiplication of intracellular bacteria by disrupting the metabolism of intracellular iron, a process which has been shown to be pH dependent, and which is crucial for the survival of certain bacteria such as L. pneumophila and F. tularensis. The growth of the fungal pathogens H. capsulatum and P. brasiliensis is also inhibited through limiting the availability of intracellular iron. C. neoformans and P. marneffei are inhibited through mechanisms independent of iron availability and are thought to be related to the disruption of other pH-dependent processes.
Bioavailability
Chloroquine is well absorbed when taken orally with a bioavailability of about 90%, with 60% being protein bound. Food has been reported to increase the bioavailability of chloroquine. Absorption may be reduced in children with protein–calorie malnutrition (kwashiorkor). In a case–control study involving five children with kwashiorkor and six healthy control subjects, peak plasma chloroquine concentrations were significantly lower in children with kwashiorkor (mean 0.04 vs 0.13 mg/ml), and the AUC was also significantly less in the malnourished children (po0.001).
Toxicity
Chloroquine is extremely well tolerated when taken for the prevention or treatment of malaria. Minor side-effects occur rarely and include nausea, vomiting and diarrhea, sleeplessness and headache and rash or pruritus. Retinal toxicity may occur following prolonged use of chloroquine but is rare with hydroxychloroquine. Neurotoxicity and cardiotoxicity have been reported but occur rarely. Importantly, though, chloroquine has an extremely narrow therapeutic range and is highly toxic in overdose.
Cardiotoxicity
Chloroquine-induced conduction disorders and cardiac myopathy have been reported in patients receiving long-term chloroquine for rheumatologic disorders. In a recent review, 46 cases of conduction disorders and 26 cases of congestive heart failure (CHF) related to chloroquine or hydroxychloroquine were described. Congestive heart failure led to death in 11 of 26 cases and cardiac transplantation in two cases. Of 13 patients with CHF who had their chloroquine/hydroxychloroquine ceased, eight improved (within three months to five years) and five died or received a transplant (within 1 week to four months). Poor outcomes were thought to be related to delayed diagnosis. Of 15 patients who had chloroquine/hydroxychloroquine ceased because of conduction disorders, only three patients showed any improvement.
Chloroquine/ hydroxychloroquine-induced cardiomyopathy is characterized by restrictive biventricular hypertrophy. Cardiac valvular thickening with mild to moderate regurgitation has also been reported. Cardiac biopsy is necessary to confirm the diagnosis. Histologic features include cellular hypertrophy of myocardiocytes with heavily vacuolated cytoplasm, and electron microscopy demonstrates curvilinear bodies within cardiac myocytes. Conduction disorders associated with chloroquine/hydroxychloroquine include bundle branch block, atrioventricular block, QRS widening, and QT prolongation. There is no evidence of clinical cardiotoxicity after oral administration of chloroquine for treatment of malaria.
- Related articles
- Related Qustion
- Mechanism of Chloroquine Jan 7, 2022
Chloroquine (CQ) and hydroxychloroquine (HCQ) are synthetic 4-aminoquinolines. CQ has been used as an antimalarial drug since World War II. Later, CQ’s use expanded to include management of systemic lupus erythematosus (SLE) and rheumatoid
- Chloroquine Nov 10, 2021
Chloroquine is an aminoquinoline used for the prevention and therapy of malaria. It is also effective in extraintestinal amebiasis and as an antiinflammatory agent for therapy of rheumatoid arthritis
Analogs of khafrefungin were designed and synthesized but none had increased potency and most had lost antifungal activity. Rustmicin is a macrolide antifungal produced by Micromonospora chalcea and Streptomyces galbus. Although not a drug....
Apr 1,2022APIAmodiaquine is a 4-aminoquinoline which has been used widely since the early 1950s to treat and prevent Plasmodium falciparum malaria. Although a 7-chloro-4-amino congener of chloroquine, it often remains effective against chloroquine-resis....
Apr 1,2022APICHLOROQUINE
54-05-7You may like
- CHLOROQUINE
-

- $1.10 / 1g
- 2022-05-14
- CAS:54-05-7
- Min. Order: 1g
- Purity: 99.9% Min
- Supply Ability: 100 Tons
- Chloroquine
-

- $0.00 / 1Kg
- 2020-04-28
- CAS:54-05-7
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 500 tons