Inorganic chemicals is the shortened form of inorganic chemical industry and is an important branch of the chemical industry with natural resources and industrial by-products as raw materials for the production of sulfuric acid, nitric acid, hydrochloric acid, phosphoric acid, soda ash, caustic soda, synthetic ammonia, fertilizer and inorganic salts, etc. This includes sulfuric acid industry, soda industry, the chloro-alkali industry, synthetic ammonia industry, fertilizer industry and mineral industry. Its broad definition also includes the production of inorganic non-metallic materials and fine inorganic product such as ceramics and inorganic pigment. The main raw material of inorganic chemical products are mineral product including sulfur, sodium, phosphorus, potassium and calcium and coal, oil, gas, and air, water and so on. Inorganic chemicals can be traced back to the ancient process of ceramics, alchemy, brewing, dyeing at thousands of years ago. Although with small scale, backward technology and pure manual manipulation, but it is the prototype of inorganic chemicals. For thousands of years, due to the low productivity, it gets slow development. Until the 18th century, it had developed rapidly. In the middle of 18th century, Britain had first applied lead chamber method using saltpeter and sulfur as raw materials to produce sulfuric acid. In 1783, Lu Bulan (France) proposed the soda method using sodium chloride, sulfuric acid, coal as raw materials. In the latter half of the 18th century, the modern chemical industry taking inorganic chemical industry as the main content had began to emerge. In 1841, people began the production of phosphate fertilizer; In 1965 Belgian Solvay realized the industrialization of ammonia soda for production of soda; with the rise of preparing potassium industry in 1870; In 1890, people began to use electrolytic approach for making Cl2 and caustic soda; In 1913, people had achieved the catalytic synthesis
What’s So Special About Platinum?
Platinum is a naturally occurring chemical element that is actually about 30 times rarer than gold, according to Jenny Luker, president of Platinum Guild International USA (PGI), a marketing organizat
Oct 14,2019 Inorganic chemistryIridium's impact
The story of iridium is both modern and prehistoric. It was discovered along with osmium in 1803 by the British chemist Smithson Tennant, born in 1761 in Selby, Yorkshire. Tennant studied medicine but
Oct 14,2019 Inorganic chemistryFacts About Iridium
Iridium is a member of the platinum family and is white in color with a yellowish hue. It has a density of 22.65 grams per cubic centimeter. By comparison, the density of lead is 11.34 g/cm3 and the d
Oct 14,2019 Inorganic chemistryProduction process and Application of Molybdenum trioxide
Molybdenum trioxide is chemical compound with the formula MoO3. This compound is produced on the largest scale of any molybdenum compound. It is an intermediate in the production of molybdenum metal.
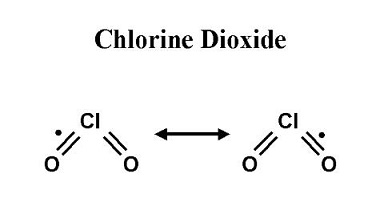
Oct 14,2019 Inorganic chemistryPreparation Method of Chlorine dioxide
Chlorine dioxide is a yellowish-green, irritating, neutral chlorine compound with the formula ClO2. It is liquefied into reddish brown liquid at 11℃, but solidified into orange red crystal at -59℃.
Oct 11,2019 Inorganic chemistryUses of Iron Oxides and Ores
Iron oxides are chemical complexes which occur naturally, comprising iron and oxygen. Here, together, 16 types of iron oxides and oxyhydroxides have been identified. These two components of oxides are
Oct 9,2019 Inorganic chemistryFacts About Osmium
Osmium was discovered in 1803 by English chemist Smithson Tennant. When separating a mixture during an experiment, he had discovered two new elements, one being osmium. The smell has been described as
Oct 9,2019 Inorganic chemistryOsmium - The World's Rarest And Densest Metal
It is unlikely that "osmium" is the first thought to enter your head upon waking. And that's a damn good thing because if so, this would not bespeak well of your mental wellbeing.
Oct 9,2019 Inorganic chemistryIndustrial uses of Ruthenium
Ruthenium combines with platinum and palladium as an effective hardener, creating alloys that are extremely wear resistant to abrasion used to make electrical contacts.
Oct 8,2019 Inorganic chemistryIndustrial Applications of Gold
Gold’s chemical and physical properties make it a very versatile element. Its noncorrosivenature provides protection as plating for other metals.
Oct 8,2019 Inorganic chemistry