| Identification | More | [Name]
Sildenafil | [CAS]
139755-83-2 | [Synonyms]
5-[2-ethoxy-5-(4-methylpiperazin-1-yl-sulphonyl)phenyl]-1-methyl-3-n-propyl-1,6-dihydro-7h-pyrazol[4,3d]pyrimidin-7-one
SILDENAFIL
SIDANAFIL
5-[2-Ethoxy-5-(4-methylpiperazin-1-yl-sulfonyl)phenyl]-1-methyl-3-n-propyl-1,6-dihydro-7H-pyrazol[4.3-d]pyrimidin-7-one
4-[2-ethoxy-5-(4-methylpiperazin-1-yl)sulfonyl-phenyl]-9-methyl-7-propyl-3,5,8,9-tetrazabicyclo[4.3.0]nona-3,7,10-trien-2-one
Sildenafil(Viagra)
5-[2-ethoxy-5-(4-methylpiperazin-1-yl-sulphonyl)phenyl]-1-methyl-3-n-propyl-1,6-dihydro-7h-pyrazol[4,3d]pyrimidin-7-one
5-(2-ETHOXY-5-(4-METHYLPIPERAZIN-1-YLSULFONYL)PHENYL)-1-METHYL-3-PROPYL-1H-PYRAZOLO[4,3-D]PYRIMIDIN-7(6H)-ONE CITRATE
5-[2-Ethoxy-5-(4-methylpiperazin-1-ylsul-fonyl)phenyl]-1-methyl-3-n-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one
viagTa
Piperazine, 1-[[3-(4,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methyl- | [EINECS(EC#)]
604-158-7 | [Molecular Formula]
C22H30N6O4S | [MDL Number]
MFCD00867528 | [Molecular Weight]
474.58 | [MOL File]
139755-83-2.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
UN1230 - class 3 - PG 2 - Methanol, solution | [WGK Germany ]
1 | [HS Code ]
38220090 | [Hazardous Substances Data]
139755-83-2(Hazardous Substances Data) |
| Questions And Answer | Back Directory | [What is sildenafil?]
Sildenafil (Viagra®) is an oral medication called a phosphodiesterase-5 (PDE5) inhibitor approved for the treatment of pulmonary arterial hypertension (PAH) in World Health Organization (WHO) Group 1 patients. The goal of this therapy is to improve exercise ability and delay clinical worsening. Research studies showing the effectiveness of the medication included mostly patients with symptoms that were rated as WHO Functional Class II-III.
Sildenafil is marketed as Revatio® for PAH and was approved by the United States Food and Drug Administration (FDA) in 2005. Sildenafil is also marketed as Viagra® which is FDAapproved for the treatment of erectile dysfunction but not for the treatment of PAH. | [Uses]
Sildenafil, the first US FDA-approved, oral phosphodiesterase type-5 inhibitor, has revolutionized the treatment of erectile dysfunction sine its approval in 1998. Since sildenafil is a potent inhibitor of cyclic guanosine monophosphate in the corpus cavernosum and therefore increases the penile response to sexual stimulation.
| [Indications]
Sildenafil Citrate is an oral drug used to treat male erectile dysfunction and is a 5-phosphodiesterase inhibitor developed by the American Pfizer Pharmaceuticals to originally treat cardiovascular disease that was later discovered to improve patients'sex lives. Sildenafil Citrate treats erectile dysfunction and was the first clinical oral drug used to specifically target male erectile dysfunction. | [Side effects]
Sildenafil is generally well tolerated. The most frequent side effects are:
Nose bleeds
Headache
Upset stomach and heartburn
Flushing of the skin
Difficulty sleeping
Worsening shortness of breath
Nasal congestion.
Other side effects include:
Fluid retention
Nausea and diarrhea
Pain in the extremity (arm or leg)
Temporary muscle aches
Fever
Numbness
A reduction in blood pressure throughout the body may occur because sildenafil relaxes blood vessels (arteries) throughout the body. Caution must be used in patients with low blood pressure, less than 90/50 mmHg for example. Caution is also needed in patients with dehydration, leftsided heart diseases and certain abnormalities of the body’s nervous system function.
Taking certain medications such as nitrates, nitric oxide donors or alpha blockers along with sildenafil can cause a significant drop in blood pressure. This could result in loss of consciousness or even death. You should make certain that you are not taking these medications before starting sildenafil. Use of sildenafil with medications known as nitrates is CONTRAINDICATED.
Prolonged erection (greater than four hours) in a male patient is a rare but very serious side effect; if this should happen to you, you should go to an emergency room or contact your doctor immediately.
Sudden loss of vision in one or both eyes has occurred in patients on PDE5 inhibitors. Such an event may represent serious dysfunction of the optic nerve and requires immediate medical attention.
Sudden loss of hearing may occur and may be accompanied by dizziness and/or ear ringing. Patients should seek prompt medical attention should this occur.
https://www.henryford.com/ | [Overdose]
Overdose information is limited. In studies with healthy volunteers, of single doses up to 800 mg, adverse events were similar to those seen at lower doses but incidence rates and severities were increased.
In cases of overdose, standard supportive measures should be adopted as required. Sildenafil blood levels are not clinically useful. Monitor ECG and blood pressure in symptomatic patients. Renal dialysis is not expected to accelerate clearance as sildenafil is highly bound to plasma proteins and not eliminated in the urine.
Contact the Poisons Information Centre on 13 11 26 for advice on the management of an overdose.
https://www.pfizer.com.au/ | [Genotoxicity]
Sildenafil was negative in in vitro bacterial and Chinese hamster ovary cell assays to detect mutagenicity, and in vitro human lymphocytes and in vivo mouse micronucleus assays to detect clastogenicity. | [Carcinogenicity]
Sildenafil was not carcinogenic when administered to rats for 24 months at a dose resulting in total systemic drug exposure (AUC) for unbound sildenafil and its major metabolite of 35- and 39-times, for male and female rats, respectively, the exposures observed in human males given the maximum recommended human dose (MRHD) of 100 mg. Sildenafil was not carcinogenic when administered to mice for 18-21 months at dosages up to the maximum tolerated dose of 10 mg/kg/day, but resulting in total systemic drug exposure for unbound sildenafil and its major metabolite of less than the exposures observed in human males given the MRHD. |
| Hazard Information | Back Directory | [Description]
Sildenafil was launched as Viagra in the US for the treatment of organic orland psychological male erectile dysfunction (ED). It is an orally bioavailable pyrazolopyrimidinone derivative structurally related to zaprinast, with vasodilating and potential anti-inflammatory activities. Upon oral administration, sildenafil selectively targets and inhibits cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5), thereby inhibiting the PDE5-mediated degradation of cGMP found in smooth muscle and increasing cGMP availability. This results in prolonged smooth muscle relaxation in the corpus cavernosum of the penis, thereby causing vasodilation, blood engorgement and a prolonged penile erection. | [Chemical Properties]
Sildenafil citrate is a white to off-white crystalline powder soluble in DMF, acetic acid and slightly soluble in methanol. Solubility of sildenafil citrate is pH dependent and it decreases with increase of pH. pH ranges between 3.7 and 3.8 and the pKa from 8.2 to 9.6. | [Originator]
Pfizer (UK) | [Definition]
ChEBI: Sildenafil is a pyrazolo[4,3-d]pyrimidin-7-one having a methyl substituent at the 1-position, a propyl substituent at the 3-position and a 2-ethoxy-5-[(4-methylpiperazin-1-yl)sulfonyl]phenyl group at the 5-position. It has a role as a vasodilator agent and an EC 3.1.4.35 (3',5'-cyclic-GMP phosphodiesterase) inhibitor. It is a pyrazolopyrimidine, a member of piperazines and a sulfonamide. | [Preparation]
The first synthetic route of sildenafil accomplished the preparation of its pyrazole derivative from ethyl 3-butyrylpyruvate and hydrazine hydrate in acetic acid, followed by the selective N-methylation of the pyrazole ring with dimethyl sulfate. The carboxylic acid was obtained after alkaline hydrolysis was subjected to nitration, followed by treatment with concentrated ammonium hydroxide solution to sequentially deliver the corresponding carboxamide derivative. The nitro group of the mentioned carboxamide derivative was then reduced to an amino group by stannous chloride/hydrochloric acid in ethanol, leading to the formation of the main 4-aminopyrazole structure. Mild amidation of the aminopyrazole derivative by the appropriate benzoyl chloride was performed, followed by cyclization mediated by hydrogen peroxide under basic environment which led to the formation of pyrimidinone heterocycle ring. Chloro-suIphonylation of pyrimidinone derivative imposed selectively on the 50 position of the phenyl ring, led to the aroyl sulfonyl chloride derivative which was then coupled with N-methylpiperazine to afford sildenafil.
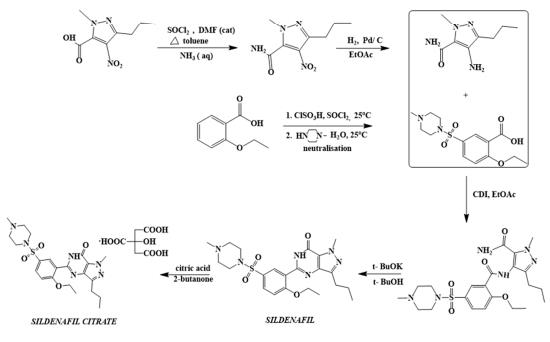
synthesis of sildenafil | [Brand name]
Viagra (Pfizer);Segurex. | [Therapeutic Function]
Vasodilator | [Mechanism of action]
Sildenafil is readily absorbed after oral administration
and reaches peak plasma levels after about an
hour. It undergoes hepatic metabolism and has a terminal
half-life of about 4 hours.An initial dose of 50 mg is
taken about an hour prior to sexual activity to induce
penile erection. | [Clinical Use]
Sildenafil is a selective inhibitor of cGMP-specific
PD-5 and therefore inhibits the degradation of cGMP.
PD-5, the predominant type in the corpus cavernosum,
also is present in other tissues (e.g., lungs, platelets, and
eye). The selective inhibition of this enzyme facilitates
the release of nitric oxide and smooth muscle relaxation
of the corpus cavernosa. Sildenafil enhances erection by
augmenting nitric oxide–mediated relaxation pathways.
It has been suggested that sildenafil’s mechanism of
action is due to cross-talk between cGMP- and cAMPdependent
transduction pathways within the cavernous
muscles. | [Enzyme inhibitor]
Sildenafil is rapidly absorbed and peaks in concentration (127–560 ng/mL) after 0.5 to 2.0 hours, displaying a half-life of 3 to 4 hours
for the full therapeutic dose (25–100 mg). It is 96% bound to plasma proteins and is metabolized by the liver CYP3A4. The
metabolite N-desmethylsildenafil possesses approximately 50% of the activity of the parent molecule. | [Drug interactions]
Potentially hazardous interactions with other drugs
Alpha-blockers: enhanced hypotensive effect - avoid
for 4 hours after sildenafil.
Antibacterials: concentration increased by
clarithromycin and erythromycin - consider
reducing sildenafil dose or frequency.
Antifungals: concentration increased by ketoconazole
- reduce initial dose for ED and avoid for PAH;
concentration increased by itraconazole - reduce
initial dose of sildenafil.
Antivirals: ritonavir significantly increases sildenafil
concentration - avoid; concentration possibly
increased by saquinavir, fosamprenavir and indinavir
- reduce dose of sildenafil; concentration reduced
by etravirine; side effects possibly increased by
atazanavir; increased risk of ventricular arrhythmias
with saquinavir - avoid; avoid with telaprevir; avoid
with tipranavir for PAH.
Cobicistat: concentration of sildenafil possibly
increased - reduce initial dose for ED and avoid for
PAH.
Nicorandil: enhanced hypotensive effect - avoid.
Nitrates: enhanced hypotensive effect - absolutely
contraindicated.
Riociguat: enhanced hypotensive effect - avoid. | [Metabolism]
In vitro metabolism studies for sildenafil have shown that the primary metabolite, N-desmethylsildenafil, and the minor metabolite, oxidative opening of the piperazine ring, are mediated by CYP3A4, CYP2C9, CYP2C19, and CYP2D6. The estimated relative contributions to clearance were 79% for CYP3A4, 20% for CYP2C9, and less than 2% for CYP2C19 and CYP2D6. These results demonstrate that CYP3A4 is the primary cytochrome mediating N-demethylation and that drugs inhibiting CYP3A4 likely impair sildenafil biotransformation and clearance. The pharmacokinetics of radiolabeled sildenafil were consistent with rapid absorption, first-pass metabolism, and primarily fecal elimination of N-demethylated metabolites. The absorption of sildenafil following oral administration was rapid (~92%), whereas the oral bioavailability was approximately 38% as a result of first-pass metabolism. | [storage]
Store at -20°C |
|