| Identification | More | [Name]
1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) | [CAS]
140681-55-6 | [Synonyms]
1-CHLOROMETHYL-4-FLUORO-1,4-DIAZONIABICYCLO[2.2.2]OCTANE BIS(TETRAFLUOROBORATE)
N-CHLOROMETHYL-N'-FLUOROTRIETHYLENEDIAMMONIUM BIS (TETRAFLUOROBORATE)
N-FLUORO-N'-(CHLOROMETHYL)TRIETHYLENEDIAMINE BIS(TETRAFLUOROBORATE)
SELECTFLUOR F-TEDA-BF4
SELECTFLUOR(R) REAGENT
SELECTFLUOR(TM) FLUORINATING REAGENT
4-diazoniabicyclo[2.2.2]octane,1-(chloromethyl)-4-fluoro-bis[tetrafluorobo
4-diazoniabicyclo[2.2.2]octane,1-(chloromethyl)-4-fluoro-bis[tetrafluoroborate(1-)]
rate(1-)]
1-chlorome.-4-fluoro-1,4-diazoniabicyclo octane bis(bf4)
F-TEDA-BF_4~SELECTFLUOR(rg Fluorinating
selectfluor fluorinating
Selectfluor
1-CHLOROME.-4-FLUORO-1,4-DIAZONIABICYCLO
SELECTFLUOR FLUORINATING REAGENT, >95% F + ACTIVE
N-Fluoro-N'-chloromethyltriethylenediaminebis(tetrafluoroborate)(SELECTFLUORTM)
1,4-Diazoniabicyclo2.2.2octane, 1-(chloromethyl)-4-fluoro-, bistetrafluoroborate(1-)
f-teda-bf_4
selectfluor fluorinating reagent
4-(chloromethyl)-1-fluoro-1,4-diazoniabicyclo[2,2,2]octane | [EINECS(EC#)]
414-380-4 | [Molecular Formula]
C7H14B2ClF9N2 | [MDL Number]
MFCD00142607 | [Molecular Weight]
354.26 | [MOL File]
140681-55-6.mol |
| Chemical Properties | Back Directory | [Appearance]
White powder | [Melting point ]
260 °C(lit.)
| [density ]
1.71 at 20℃ | [vapor pressure ]
0.001Pa at 25℃ | [storage temp. ]
2-8°C
| [solubility ]
Acetonitrile (Slightly), DMSO (Slightly), Methanol (Very Slightly, Heated), Water | [form ]
Powder | [color ]
White | [Water Solubility ]
soluble | [Sensitive ]
Moisture Sensitive | [Merck ]
8425 | [BRN ]
5368649 | [InChIKey]
ZQGDSZPGKPJABN-UHFFFAOYSA-N | [LogP]
-3.39 at 20℃ | [Surface tension]
73.1mN/m at 1g/L and 19℃ | [CAS DataBase Reference]
140681-55-6(CAS DataBase Reference) | [EPA Substance Registry System]
140681-55-6(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,O,F,Xi | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin .
R41:Risk of serious damage to eyes. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RIDADR ]
UN 3088 4.2/PG 2
| [WGK Germany ]
2
| [Hazard Note ]
Oxidising Agent | [TSCA ]
T | [HazardClass ]
8 | [PackingGroup ]
II | [HS Code ]
29335990 | [Toxicity]
LD50 in male rats: 640 mg/kg orally; >2 g/kg dermally (Banks, 1998) |
| Questions And Answer | Back Directory | [Tetrafluoroborate]
1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate), with the formula C7H14B2ClF9N2, is a selective fluorine reagent which is white crystal at room temperature. It is mainly used asfluorinating agent to make single fluoridation with electronic double bond, silyl enol ether and enol lithium salt and so on. It can be used in the preparation of fluorinated steroid drugs, etc. | [Reaction]
1. It is one of the most efficient and popular reagents for electrophilic fluorination. The product is also useful as a reagent, or catalyst, for oxidative transformations, coupling reactions and halogenations.
2. Useful for the direct electrophilic fluorination of ketones, ketals and enamides.
3. Ritter-type C-H amination of unactivated sp3.
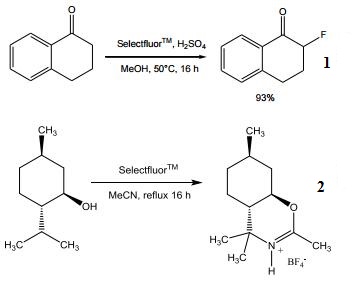
| [Main Usage]
It is mainly used as fluorinating agent to make single fluoridation with electronic double bond, silyl enol ether and enol lithium salt and so on. It can be used in the preparation of fluorinated steroid drugs, etc. |
| Hazard Information | Back Directory | [Description]
1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate)(Selectfluor) is a white solid (mp 190 °C), stable in air and to moisture. It is a dicationic salt soluble in cold water or dilute hydrochloric acid. However, it decomposes in dilute sodium hydroxide and reacts with cold DMSO (rapidly and exothermally) and with DMF (slowly on heating). It is moderately soluble in acetonitrile but only slightly soluble in lower alcohols and acetone, which makes the former the solvent of choice. Occasionally, reactions in these solvents were accelerated by adding a small amount of water or trifluoroacetic acid. Bulk quantities of Selectfluor should be stored in a cool, dry place and not heated above 80 °C. Fainzil’berg et al. have measured the reduction potentials of Selectfluor and other N-F compounds. With a positive reduction potential, E1/2 ) 0.33 V, Selectfluor is predicted to be a more reactive fluorinating reagent than, e.g., N-fluorobenzenesulfonimide, with E1/2 ) -1.24 V[1].
| [Chemical Properties]
White powder | [Uses]
A highly effective and versatile source of electrophilic fluorine. | [Uses]
Effective electrophilic fluorinating reagent for a wide variety of substrates such as aromatics, alkenes, carbohydrates, etc. | [Uses]
Mild, versatile fluorinating reagent used in pharmaceutical and agricultural applications. | [General Description]
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product is a fluorine donor also called Selectfluor and has been enhanced for catalysis. Find details here. | [reaction suitability]
reagent type: catalyst
reagent type: oxidant
reaction type: C-H Activation | [Toxicology]
1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) (F-TEDA-BF4) is moderately toxic (male rat oral LD50 640 mg kg?1; female rat 350–500 mg kg?1), and is an irritant to the eye and respiratory system. Always use approved dust mask (or respirator), gloves, and safety glasses when handling the solid. Carry out solubility tests on a sensible scale when seeking alternative solvents (note the problem with DMSO referred to above).
| [Advantages]
As the advantages of non-toxicity, non-corrosion and chemical stability, 1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) have been widely used in industry and public life.
| [References]
[1] Rajendra P. Singh, Jean’ne M. Shreeve. “Recent Highlights in Electrophilic Fluorination with 1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane Bis(tetrafluoroborate).” ChemInform 35 16 (2004).
|
|
|