| Identification | More | [Name]
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide | [CAS]
1892-57-5 | [Synonyms]
1-(3-DIMETHYLAMINOPROPYL)-3-ETHYLCARBODIIMIDE
1-ETHYL-3-(3-DIMETHYLAMINOPROPYL) CARBODIIMIDE
EDAC
EDC
EDCI
N-(3-DIMETHYLAMINOPROPYL)-N'-ETHYLCARBODIIMIDE
N-ETHYL-N'-(3-DIMETHYLAMINOPROPYL)CARBODIIMIDE
WATER-SOLUBLE CARBODIIMIDE
WSCD
WSCI
[3-(Dimethylamino)propyl]ethylcarbodiimide
ethyldimethylaminopropylcarbodiimide
n’-(ethylcarbonimidoyl)-n,n-dimethyl-3-propanediamine
N3-Ethyliminomethylene-N1,N1-dimethylpropane-1,3-diamine
1-(3-dimethylaminopropyl)-N?ethylcarbodiimide
N'-(ethylcarbonimidoyl)-N,N-dimethylpropane-1,3-diamine
EDC polymer-bound, N-Ethyl-Nμ-(3-dimethylaminopropyl)carbodiimide polymer-bound, N-(3-Dimethylaminopropyl)-Nμ-ethylcarbodiimide polymer-bound
EDC, WSC, N-Ethyl-Nμ-(3-dimethylaminopropyl)carbodiimide
3-[(Ethylcarbonimidoyl)amino]-N,N-dimethyl-1-propanamine
WSC【Dotite】 | [EINECS(EC#)]
217-579-2 | [Molecular Formula]
C8H17N3 | [MDL Number]
MFCD00044916 | [Molecular Weight]
155.24 | [MOL File]
1892-57-5.mol |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 2735 8/PG 3
| [WGK Germany ]
3
| [F ]
1-3-10 | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
III | [HS Code ]
29252900 |
| Hazard Information | Back Directory | [Description]
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide is a water-soluble carbodiimide available as hydrochloride salt. This compound is a carboxyl- and amine-reactive crosslinker that is water soluble and can activate carboxyl residues for their reaction with primary amines to form O-acylisourea as a reaction intermediate, which reacts with amide bonds to release isourea as a by-product. It can crosslink the carboxy-group of glutamate and aspartate to the side-chain primary amine of lysine and the amino group at the N-terminus. The crosslinker readily reacts at a pH range of 4.5–7.2 and only crosslinks the residues within their van der Waals distance. 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide is also widely used in fabricating different nanosystems implemented in drug/protein delivery systems. It is mainly used for surface crosslinking/immobilization of drugs to increase stability against environmental changes, such as pH or temperature[1-2].
| [Chemical Properties]
Clear light yellow liquid | [Uses]
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide is used as a carboxyl activating agent and activate phosphate groups in phospho mono and di esters. It is used in peptide synthesis, 3'-amino-3'-deoxyadenosine-5'-di- and triphosphates and in the preparation of antibodies like immunoconjugates. It plays a vital role for immobilization of large biomolecules in association with N-hydroxysuccinimide. It is also used in the acylation of phosphoranes. | [General Description]
N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide is commonly used in combination with N-hydroxysuccinimide (NHS) in carbodiimide coupling reaction to activate carboxyl group for coupling with amines to form amides. | [reaction suitability]
reaction type: Coupling Reactions | [Synthesis]
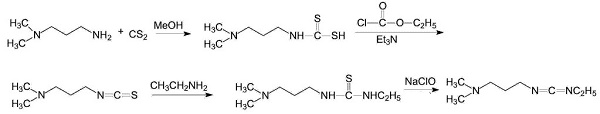
The production method of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide is to react with N, N'-dimethylpropanediamine and carbon disulfide as raw materials to prepare N, N'- Dimethylpropylthiourea. Then it is oxidized with ethyl chloroformate to obtain N, N'-dimethylpropyl isothiocyanate, which is then reacted with ethylamine and oxidized with sodium hypochlorite to obtain the product 1-(3-dimethylaminopropyl)-3- Ethylcarbodiimide.
| [References]
[1] Manjula ;Mummadisetti et al.“Chapter Five-An approach to nearest neighbor analysis of pigment-protein complexes using chemical cross-linking in combination with mass spectrometry.” Methods in enzymology" Methods in Enzymology, 680 (2023) 139-162.
[2] Dmour, Isra and M. Taha. “Natural and semisynthetic polymers in pharmaceutical nanotechnology.”Organic Materials as Smart Nanocarriers for Drug Delivery (2018) 35-100.
|
|
|