| Identification | More | [Name]
TOCAINIDE | [CAS]
41708-72-9 | [Synonyms]
TOCAINIDE
2-amino-2',6'-propionoxylidide
2-amino-n-(2,6-dimethylphenyl)-propanamid
6'-propionoxylidide, 2-amino-2
alanyl-2,6-xylidide
Astra W-36095
W-36095 | [EINECS(EC#)]
255-505-0 | [Molecular Formula]
C11H16N2O | [MDL Number]
MFCD00072010 | [Molecular Weight]
192.26 | [MOL File]
41708-72-9.mol |
| Chemical Properties | Back Directory | [Melting point ]
246-266 °C | [Boiling point ]
328.25°C (rough estimate) | [density ]
1.0529 (rough estimate) | [refractive index ]
1.5750 (estimate) | [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [form ]
Solid | [pka]
pKa 7.75(H2O
t = 25.0±0.2
I = 0.01
(NaCl)) (Uncertain) | [color ]
White to off-white | [CAS DataBase Reference]
41708-72-9(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Originator]
Tonocard,Astra,UK,1981 | [Uses]
Tocainide is used for suppressing symptoms of ventricular arrhythmia and tachycardia,
and for premature cardiac contractions. | [Definition]
ChEBI: A monocarboxylic acid amide in which 2,6-dimethylphenylaniline and isobutyric acid have combined to form the amide bond; used as a local anaesthetic. | [Manufacturing Process]
The compound 2-amino-2',6'-propionoxylidide was synthesized by saturating
with gaseous ammonia at room temperature a suspension of 50 g (0.195 mol)
of 2-bromo-2',6'-propionoxylidide in a mixture of 500 ml of 95% alcohol and
400 ml of concentrated aqueous ammonia. The saturation was carried out
under mechanical stirring. After 25 hours the mixture was resaturated with
ammonia gas. The stirring at room temperature was continued for a total
period of 116 hours, and a sample was taken at that time. Gas
chromatographic analysis indicated that about 95% of the bromo compound
had been converted to the desired product.
The solvents were evaporated in vacuo, and the residue was taken up in 80
ml of 3 M hydrochloric acid. After addition of 220 ml of water, the insoluble
material was filtered off, washed with 100 ml of water and then dried. The
insoluble material weighed 9.5 g and was mainly unreacted bromo compound. The filtrate was reacted with 50 ml of 7 M NaOH, extracted three times with
methylene chloride (50 ml + 2 x 25 ml portions), dried over potassium
carbonate, and then evaporated. The yield of residue was 26.8 g which
corresponds to 71.4% of the theoretical yield. This residue was a colorless
solidifying oil and was dissolved in 200 ml chloroform. Hydrogen chloride was
bubbled in until a sample of the solution tested acidic to wet pH indicator
paper. A precipitate was obtained and recovered by filtration. The precipitate
was washed with chloroform and dried. The melting point was determined to
be from 246°C to 247.5°C. | [Brand name]
Apx;Citocard;Taquidil;Tonocard;Toquidil;Xylotocan. | [Therapeutic Function]
Antiarrhythmic | [World Health Organization (WHO)]
Tocainide, an antidysrhythmic agent, was introduced in 1981 for
the treatment of ventricular dysrhythmias. By 1984 its use was associated with
cases of agranulocytosis, aplastic anaemia and thrombocytopenia, some of which
were fatal. This led some regulatory authorities to restrict the indications for its
use. The major manufacturer has subsequently restricted its use on a worldwide
basis to the treatment of symptomatic ventricular dysrhythmias not responding to
other therapy, or when other therapy is contraindicated. | [Pharmacokinetics]
The α-methyl group is believed to slow the rate of metabolism and, thereby, to
contribute to oral activity. The plasma half-life of tocainide is approximately 12 hours, and nearly
50% of the drug may be excreted unchanged in the urine. Adverse effects associated with tocainide
are like those observed with lidocaine—specifically, gastrointestinal disturbances and central
nervous system effects. | [Clinical Use]
Tocainide (Tonocard) is an orally effective antiarrhythmic
agent with close structural similarities to lidocaine. Tocainide is indicated for the treatment of symptomatic
ventricular arrhythmias refractory to more conventional
therapy. Serious noncardiac adverse effects limit its use
to patients with life-threatening arrhythmias. | [Side effects]
Light-headedness, dizziness, or nausea occurs in approximately
15% of patients, paresthesias and numbness
in 9%, and tremor in 8%.These adverse effects are
generally mild in intensity, transient, and dose related.
Overall, however, approximately 20% of patients prescribed
tocainide discontinue therapy because of such
effects. Serious immune-based side effects, such as pulmonary
fibrosis, have been reported, and blood
dyscrasias, such as agranulocytosis and thrombocytopenia,
may occur in up to 0.2% of patients. | [Synthesis]
Tocainide, 2-amino-2�,6�-dimethylpropionanilide (18.1.8), is synthesized by
reacting 2,6-dimethylaniline with 2-bromopropionic acid bromide and subsequent substitution
of the bromine atom in the resulting amide (18.1.7) with an amino group.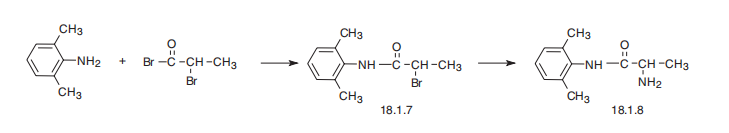
| [Drug interactions]
When used with other class IB antiarrhythmic drugs, tocainide
toxicity may be increased without significant
gain in antiarrhythmic efficacy. | [Precautions]
Patients who are hypersensitive to tocainide or to local
anesthetics of the amide type should not be exposed to
tocainide.The presence of second- or third-degree heart
block in the absence of an artificial pacemaker also contraindicates
the use of tocainide. |
|
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
| Company Name: |
DC Chemicals
|
| Tel: |
021-58447131 13564518121 |
| Website: |
https://www.chemicalbook.com/ShowSupplierProductsList927327/0.htm |
|