| Identification | More | [Name]
6-Bromoquinoline | [CAS]
5332-25-2 | [Synonyms]
6-BROMOQUINOLINE
TIMTEC-BB SBB001559
6-bromo-quinolin
Quinoline, 6-bromo-
6-BROMOQUINOLINE 95+%
6-BROMOOQUINOLINE
6-Bromoquinoline ,98% | [EINECS(EC#)]
206-363-3 | [Molecular Formula]
C9H6BrN | [MDL Number]
MFCD00024023 | [Molecular Weight]
208.05 | [MOL File]
5332-25-2.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R41:Risk of serious damage to eyes.
R37/38:Irritating to respiratory system and skin .
R22:Harmful if swallowed.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection .
S39:Wear eye/face protection .
S36:Wear suitable protective clothing . | [WGK Germany ]
3 | [Hazard Note ]
Irritant | [TSCA ]
Yes | [HazardClass ]
IRRITANT | [HS Code ]
29334900 |
| Hazard Information | Back Directory | [Chemical Properties]
Thick Oil | [Uses]
6-Bromoquinoline is used as fine chemical and pharmaceutical intermediate, used as the coupling reagent. | [Synthesis]
4-Bromobenzenamine (25 g, 145.32 mmol, 1.00 equiv), sodium 3-nitrobenzenesulfonate (55.5 g, 246.64 mmol, 1.70 equiv), propane-1,2,3-triol (50.8 g, 551.63 mmol, 3.80 equiv), and sulfuric acid (170 mL, 70%) were placed into a 500-mL round-bottom flask. The resulting solution was stirred overnight at 140° C. The pH value of the solution was adjusted to with 10% aqueous sodium hydroxide. The resulting solution was extracted with 5*150 mL of ethyl acetate. The organic layers were combined and dried over anhydrous sodium sulfate, and concentrated in vacuo. The residue was purified by silica gel column chromatography eluting with ethyl acetate/petroleum ether (1:50). This resulted in 17.2 g (42%) of 6-bromoquinoline as yellow oil. |
| Questions And Answer | Back Directory | [Bromoquinoline]
There are seven positional isomers of bromoquinoline and their main properties are listed below:
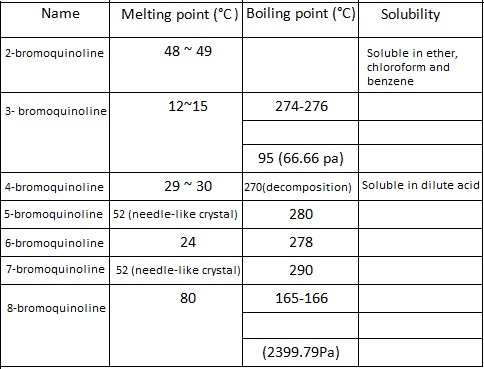
| [Application and synthetic method]
3-bromo-quinoline is reacted with mixed acid for generating 3-bromo-5-nitro-quinoline, which heats together with potassium permanganate for being oxidation into 5-bromo-2, 3-pyridine dicarboxylic acid.
6-bromo-quinoline is heated together with nitric acid to generate 6-bromo-8-nitro quinolone with further reaction with potassium permanganate for being oxidized to 2, 3-pyridinedicarboxylic acid.
2-bromo-quinolien can be synthesized through the reaction between 2-hydroxy quinoline and phosphorus pentabromide.
Quinoline perbromide is heated at 180 °C for generating 3-bromo-quinoline.
From the heating between 4-hydroxy quinoline and phosphorus pentabromide, or from the diazotization reaction via 4-aminoquinoline to generate 4-bromo-quinoline;
5-bromo-quinoline is synthesize by the heating reaction between m-bromo-aniline, glycerol, m-bromo nitrobenzene and concentrated sulfuric acid, or from the diazotization reaction of 5-aminoquinoline.
6-bromo-quinoline can be synthesized from the heating of bromoaniline, glycerol, concentrated sulfuric acid, and p-bromo-nitrobenzene.
7-bromo-quinoline can be synthesized from the diazotization reaction of 7-aminoquinoline.
8-bromo-quinoline can be synthesized from the heating reaction of o-bromo-aniline, glycerol, concentrated sulfuric acid and o-bromo nitrobenzene.
application: as organic synthesis reagents.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
|
|
|