| Identification | More | [Name]
Lithium carbonate | [CAS]
554-13-2 | [Synonyms]
CARBONIC ACID DILITHIUM SALT
CARBONIC ACID LITHIUM SALT
Dilithium acrbonate
DILITHIUM CARBONATE
LITHIUM
LITHIUM AA SINGLE ELEMENT STANDARD
LITHIUM, AAS STANDARD SOLUTION
LITHIUM AA STANDARD
LITHIUM ATOMIC ABSORPTION SINGLE ELEMENT STANDARD
LITHIUM ATOMIC ABSORPTION STANDARD
LITHIUM ATOMIC ABSORPTION STANDARD SOLUTION
LITHIUM ATOMIC SPECTROSCOPY STANDARD
Lithium carboate
LITHIUM CARBONATE
LITHIUM CARBONATE SOLUTION
LITHIUM ICP/DCP STANDARD
LITHIUM ICP STANDARD
LITHIUM IC STANDARD
LITHIUM METAL
LITHIUM METALLO-ORGANIC STANDARD | [EINECS(EC#)]
209-062-5 | [Molecular Formula]
CLi2O3 | [MDL Number]
MFCD00134051 | [Molecular Weight]
73.89 | [MOL File]
554-13-2.mol |
| Chemical Properties | Back Directory | [Appearance]
Lithium carbonate is a white hygroscopic powder. | [Melting point ]
720 °C | [Boiling point ]
1342 °C(lit.)
| [bulk density]
250kg/m3 | [density ]
2.11 g/mL at 25 °C
| [Fp ]
1310°C | [storage temp. ]
Store at +5°C to +30°C. | [solubility ]
13g/l | [form ]
wire
| [pka]
pKa 6.38 (Uncertain);10.25 (Uncertain) | [color ]
White | [Specific Gravity]
2.11 | [Flame Color]
Blue | [Odor]
odorless | [PH]
10-11 (5g/l, H2O, 20℃) | [Water Solubility ]
13 g/L (20 ºC) | [Merck ]
14,5527 | [BRN ]
3999191 | [Solubility Product Constant (Ksp)]
pKsp: 1.6 | [BCS Class]
1 | [InChIKey]
XGZVUEUWXADBQD-UHFFFAOYSA-L | [LogP]
-0.809 (est) | [CAS DataBase Reference]
554-13-2(CAS DataBase Reference) | [NIST Chemistry Reference]
Lithium carbonate(554-13-2) | [EPA Substance Registry System]
554-13-2(EPA Substance) |
| Questions And Answer | Back Directory | [Description]
Lithium carbonate (molecular structure is Li2CO3, English name is lithium carbonate) as a colorless monoclinic crystal or white powder. Density is 2.11. Melting point is 618 ℃. Without deliquescence, it is stable in the air. Low solubility in water, the solubility decreases with increasing temperature. Solubility in cold water is greater than hot water. It is Soluble in dilute acid, insoluble in alcohol and acetone. Carbon dioxide is introduced into the aqueous suspension of lithium carbonate, lithium carbonate is converted to lithium acid carbonate and dissolved. If the solution of lithium acid carbonate is heated and then it releases carbon dioxide and precipitates lithium carbonate. The nature of the lithium carbonate may be used to remove impurities from lithium carbonate. Since lithium ion has a strong polarizability, thus thermal stability of lithium carbonate is worse than other alkali metal carbonate, when heated to above the melting point, it will decompose to produce carbon dioxide and lithium oxide.
 | [Chemical Properties]
Lithium carbonate is a white monoclinic crystalline solid. Typically for carbonates, lithium carbonate reacts with acids stronger than carbon dioxide or carbonic acid to yield the lithium salt of the acid and carbon dioxide. The reactions may be carried out in a solution, as an aqueous slurry, or, less effectively, with solid lithium carbonate.
Lithium carbonate exhibits a low water solubility for an alkali metal carbonate. The solubility decreases with increasing temperature. It is not hygroscopic and is generally stable when exposed to the atmosphere. In fact, it is the normal end compound encountered when many basic lithium compounds are exposed to the atmosphere. Lithium carbonate may be dissolved in water by conversion to the hydrogen carbonate. Releasing carbon dioxide by heating the solution of lithium hydrogen carbonate causes reprecipitation of the lithium carbonate.
| [Uses]
The lithium carbonate industry is a global high monopoly industry, the current production capacity is mainly concentrated in three foreign manufacturers of SQM, FMC, Chemetall and so on.
Industrial lithium carbonate is used in the manufacture of other lithium salts, such as lithium chloride and lithium bromide and so on. It also acts as lithium oxide materials in enamel, glass, pottery and porcelain enamel, and it is also added to the electrolytic cell for electrolysis of aluminum to increase the current efficiency and reduce the internal resistance of the cell and the bath temperature. In medicine, it is mainly used for the treatment of mania, can improve their emotional disorders for schizophrenia. It has the effect of elevating peripheral leukocytes; can be used for synthetic rubber, dyes, semiconductor and military defense industry and so on; for the production of lithium tantalate, lithium niobate and other acoustic grade single crystal, optical grade monocrystalline etc; for preparation of the acoustic grade single crystal.
Battery grade lithium carbonate is mainly used for the preparation of lithium cobalt oxide, lithium manganese oxide, ternary materials, lithium iron phosphate and other lithium ion battery cathode materials; used in a matrix modifier; as aneuroprotective effect of lithium carbonate in amyotrophic lateral sclerosis.
| [Toxicity]
Lithium carbonate has a significant stimulating effect, firstly has damage on the gastrointestinal tract, kidney and central nervous system. Toxicity order of lithium compounds is Li <LiCl <Li2CO3, maximum allowable concentration: Lithium condensation and fragmentation aerosol were 0.05 mg/m3 and 0.5 mg/m3.
Wear rubber gloves and protective masks when working, in order to protect the respiratory organs against dust.
| [Preparation]
Lithium carbonate is obtained as an intermediate product in recovery of lithium metal from its ore, spodumene (See Lithium). It is prepared by mixing a hot and concentrated solution of sodium carbonate with lithium chloride or sulfate solution.
Li2SO4+ Na2CO3→Li2CO3+ Na2SO4 | [Reactions]
Lithium carbonate reacts with dilute acids, liberating carbon dioxide:
Li2CO3+ HCl →LiCl + CO2+ H2O
Thermal decompostion yields lithium oxide and carbon dioxide:
Li2CO3 → Li2O + CO2
Reaction with lime produces lithium hydroxide:
Li2CO3+ Ca(OH)2→2LiOH + CaCO3
The carbonate reacts with molten aluminum fluoride converting to lithium fluoride:
3Li2CO3+ 2AlF3 → 6LiF + 3CO2+ Al2O3
It combines with carbon dioxide in aqueous slurry forming soluble bicarbonate, which decomposes to carbonate upon heating:
Li2CO3+ CO2+ H2O →2LiHCO3
The bicarbonate can not be separated in solid form. It exists only in solution when carbonate dissolves in water saturated with CO2under pressure.
|
| Safety Data | Back Directory | [Hazard Codes ]
Xn,C,F | [Risk Statements ]
R36/38:Irritating to eyes and skin .
R41:Risk of serious damage to eyes.
R36/37/38:Irritating to eyes, respiratory system and skin .
R22:Harmful if swallowed.
R36:Irritating to the eyes.
R34:Causes burns.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R15:Contact with water liberates extremely flammable gases.
R14:Reacts violently with water.
R11:Highly Flammable. | [Safety Statements ]
S8:Keep container dry .
S43:In case of fire, use ... (indicate in the space the precise type of fire-fighting equipment. If water increases the risk add-Never use water) .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37:Wear suitable protective clothing and gloves .
S24/25:Avoid contact with skin and eyes .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S16:Keep away from sources of ignition-No smoking .
S7/8:Keep container tightly closed and dry . | [WGK Germany ]
2
| [RTECS ]
OJ5800000
| [F ]
10 | [TSCA ]
Yes | [HS Code ]
28369100 | [Safety Profile]
Human carcinogenic
data. Poison by intraperitoneal and
intravenous routes. Moderately toxic by
ingestion and subcutaneous routes. Human
systemic effects by ingestion: toxic
psychosis, tremors, changes in fluid intake,
muscle weakness, increased urine volume,
nausea or vomiting, allergic dermatitis.
Human reproductive effects by ingestion:
effects on newborn, including Apgar score
changes and other neonatal measures or
effects. Human teratogenic effects by
ingestion: developmental abnormalities of
the cardiovascular system, central nervous
system, musculoskeletal and gastrointestinal
systems. An experimental teratogen.
Experimental reproductive effects.
Experimental carcinogen producing
leukemia and thyroid tumors. Human
mutation data reported. Used in the
treatment of manic-depressive psychoses.
Incompatible with fluorine. See also
LITHIUM COMPOUNDS. | [Hazardous Substances Data]
554-13-2(Hazardous Substances Data) |
| Hazard Information | Back Directory | [General Description]
A white powder. Strong irritant when dissolved in water. | [Reactivity Profile]
A base. Decomposed by acids with the evolution of carbon dioxide. Fluorine burns fiercely on contact with LITHIUM CARBONATE(554-13-2). | [Air & Water Reactions]
Slightly soluble in water. | [Potential Exposure]
Lithium carbonate is used in treatment
of manic-depressive psychoses; to make ceramics and porcelain glaze; varnishes, dyes, pharmaceuticals, coating of
arc-welding electrodes; battery alloys; nucleonics, luminescent paints; glass ceramics; lubricating greases; in aluminum production | [First aid]
Move victim to fresh air. Call 911 or emergency
medical service. Give artificial respiration if victim is not
breathing. Do not use mouth-to-mouth method if victim
ingested or inhaled the substance; give artificial respiration
with the aid of a pocket mask equipped with a one-way
valve or other proper respiratory medical device.
Administer oxygen if breathing is difficult. Remove and
isolate contaminated clothing and shoes. In case of contact
with substance, immediately flush skin or eyes with running water for at least 20 minutes. For minor skin contact,
avoid spreading material on unaffected skin. Keep victim
warm and quiet. Effects of exposure (inhalation, ingestion,
or skin contact) to substance may be delayed. Ensure that
medical personnel are aware of the material(s) involved
and take precautions to protect themselves. Medical observation is recommended for 24 to 48 hours after breathing
overexposure, as pulmonary edema may be delayed. As
first aid for pulmonary edema, a doctor or authorized
paramedic may consider administering a drug or other
inhalation therapy | [Shipping]
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required | [Incompatibilities]
The aqueous solution is a strong base.
Reacts violently with acids, powdered calcium and fluorine.Incompatible with oxidizers (chlorates, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep away
from alkaline materials, strong acids, powdered calcium,
fluorine, moisture. Corrodes aluminum, copper, zinc. | [Physical properties]
White monoclinic crystals; refractive index 1.428; density 2.11 g/cm3; melts at 723°C; decomposes at 1,310°C; low solubility in water (1.54 g/100g) at 0°C; 1.32 g//100g at 20°C), solubility decrease with temperature (0.72g/100g at 100°C); insoluble in acetone and ethanol. | [Definition]
lithium carbonate: A white solid,Li2CO3; r.d. 2.11; m.p. 723°C; decomposesabove 1310°C. It is producedcommercially by treating the ore with sulphuric acid at 250°C andleaching the product to give a solutionof lithium sulphate. The carbonateis then obtained by precipitationwith sodium carbonate solution.Lithium carbonate is used in the preventionand treatment of manicdepressivedisorders. It is also usedindustrially in ceramic glazes. | [Indications]
Lithium inhibits thyroidal incorporation of I- into Tg, as
well as the secretion of thyroid hormones, but it does
not inhibit the activity of the Na+-I- symporter or the
accumulation of I- within the thyroid. Lithium offers no
particular advantage over drugs of the thionamide class
but may be employed for temporary control of thyrotoxicosis
in patients who are allergic to both thionamides
and iodide. | [Brand name]
Eskalith (GlaxoSmithKline); Lithane (Bayer); Lithobid
(JDS); Lithonate (Solvay Pharmaceuticals). | [Flammability and Explosibility]
Nonflammable | [Clinical Use]
Treatment and prophylaxis of mania, manic
depressive illness, and recurrent depression
Aggressive or self-mutilating behaviour | [Side effects]
Drowsiness, dizziness, tiredness, increased thirst, increased frequency of urination, weight gain, and mildly shaking hands (fine tremor) may occur. These should go away as your body adjusts to the medication.
This medication may increase serotonin and rarely cause a very serious condition called serotonin syndrome/toxicity. The risk increases if you are also taking other drugs that increase serotonin, so tell your doctor or pharmacist of all the drugs you take (see Drug Interactions section). Get medical help right away if you develop some of the following symptoms: fast heartbeat, hallucinations, loss of coordination, severe dizziness, severe nausea/vomiting/diarrhea, twitching muscles, unexplained fever, unusual agitation/restlessness.
| [Synthesis]
Lithium carbonate is synthesized by reacting lithium salts with soda
or potash, followed by purification of the salt, which is not readily soluble [75].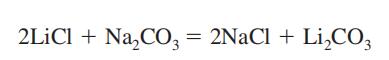
| [Drug interactions]
Potentially hazardous interactions with other drugs
ACE inhibitors and angiotensin-II antagonists:
lithium excretion reduced - avoid.
Analgesics: NSAIDs and ketorolac reduce excretion
of lithium.
Anti-arrhythmics: increased risk of ventricular
arrhythmias with amiodarone - avoid.
Antidepressants: increased risk of CNS effects
with SSRIs; risk of toxicity with tricyclics; possible
increased serotonergic effects with venlafaxine.
Antipsychotics: increased risk of extrapyramidal side
effects and possibly neurotoxicity with clozapine,
flupentixol, haloperidol, phenothiazines, risperidone
or zuclopenthixol; increased risk of extrapyramidal
side effects with sulpiride; possible risk of toxicity
with olanzapine.
Cytotoxics: increased risk of ventricular arrhythmias
with arsenic trioxide.
Dapoxetine: increased risk of serotonergic effects -
avoid.
Diuretics: lithium excretion reduced by loop
diuretics, potassium-sparing diuretics, aldosterone
antagonists and thiazides; lithium excretion
increased by acetazolamide.
Methyldopa: neurotoxicity may occur without
increased lithium levels. | [Metabolism]
Lithium is excreted mainly unchanged in the urine; only
a small amount can be detected in the faeces, saliva, and
sweat. | [storage]
Store at RT | [Purification Methods]
Crystallise it from water. Its solubility decreases as the temperature is raised. The solubility in H2O is 1.3% at ~10o, and 0.7% at ~100o. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 987 1963, Caley & Elving Inorg Synth I 1 1939.] |
|
|