| Identification | More | [Name]
Midazolam maleate salt | [CAS]
59467-94-6 | [Synonyms]
8-CHLORO-6-[2-FLUOROPHENYL]-2-METHYL-4H-IMIDAZO[1,5-A]-[1,4]BENZODIAZEPINE MALEATE SALT
MIDAZOLAM MALEATE SALT
5-a)(1,4)benzodiazepine,8-chloro-6-(2-fluorophenyl)-1-methyl-4h-imidazo((z
8-chloro-6-(2-fluorophenyl)-1-methyl-4h-imidazo(1,5-a)(1,4)benzodiazepinemal
dormicum
dormicummaleate
midazolammaleate
ro21-3981/001
4H-Imidazo[1,5-a][1,4]benzodiazepine, 8-chloro-6-(2-fluoro-phenyl)-1-methyl-, (Z)-2-butenedioate (1:1)
midazolam maleate--dea schedule iv item
8-chloro-6-(2-fluorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepine monomaleate
DL-A-LYSOPHOSPHATIDYLCHOLINE-*GAMMA-O-HE XADECYL
4H-Imidazo1,5-a1,4benzodiazepine, 8-chloro-6-(2-fluorophenyl)-1-methyl-, (2Z)-2-butenedioate (1:1)
8-chloro-6-(2-fluorophenyl)-1-methyl-4h-imidazo[1,5-a][1,4]benzodiazepine maleate salt
8-Chloro-6-(2-fluorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepine maleate
Ro-21-3981 | [EINECS(EC#)]
261-775-0 | [Molecular Formula]
C22H17ClFN3O4 | [MDL Number]
MFCD00941440 | [Molecular Weight]
441.84 | [MOL File]
59467-94-6.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed. | [Safety Statements ]
S36:Wear suitable protective clothing . | [RIDADR ]
3249 | [WGK Germany ]
3
| [RTECS ]
NI2922200
| [HazardClass ]
6.1(b) | [PackingGroup ]
III | [Toxicity]
LD50 in male mice (mg/kg): 760 orally; 86 i.v. (Pieri) |
| Hazard Information | Back Directory | [Description]
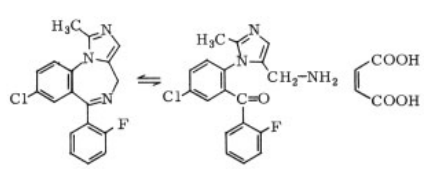
Midazolam maleate, 8-
chloro-6-(2-fluorophenyl)?1-methyl-4Himidazo-benzodiazepine maleate
, is a
stable, water-soluble powder. The solubility in
water depends on pH:≈85 mg/mL at pH 2.7 and
0.3 mg/mL at pH 7.6. The maleate is subject to
reversible ring opening. Below pH4 the ring is
open; above pH 4 the cyclic form is present. The
anesthetic formulation is a buffered aqueous
solution containing 2.5 mg/mL at pH 3.5. | [Originator]
Dormicum,Roche,Switz.,1982 | [Uses]
Sedative; ligand GABA receptor benzodiazepine modulatory site | [Manufacturing Process]
Acetic anhydride (7 ml) was added to a solution of 6.16 g of crude 2-
aminomethyl-7-chloro-2,3-dihydro-5-(2-fluorophenyl)-1H-1,4-benzodiazepine
in 200 ml of methylene chloride. The solution was added to 200 ml of
saturated aqueous sodium bicarbonate and the mixture was stirred for 20
minutes. The organic layer was separated, washed with sodium bicarbonate,
dried over sodium sulfate and evaporated to leave resinous 2-
acetylaminomethyl-7-chloro-2,3-dihydro-5-(2-fluorophenyl)-lH -l,4-
benzodiazepine. This material was heated with 40 g of polyphosphoric acid at
150°C for 10 minutes. The cooled reaction mixture was dissolved in water,
made alkaline with ammonia and ice and extracted with methylene chloride.
The extracts were dried and evaporated and the residue was
chromatographed over 120 g of silica gel using 20% methanol in methylene
chloride. The clean fractions were combined and evaporated to yield resinous
8-chloro-3a,4-dihydro-6-(2-fluorophenyl)-1- methyl-4H-imidazo[1,5-a][1,4] -
benzodiazepine.
A mixture of this material with 500 ml of toluene and 30 g of manganese
dioxide was heated to reflux for 1? hours. The manganese dioxide was
separated by filtration over Celite. The filtrate was evaporated and the residue
was crystallized from ether to yield 8-chloro-6-(2-fluorophenyl)-1-methyl-4H�imidazo[1,5-a][1,4]benzodiazepine, melting point 152°C to 154°C. The
analytical sample was recrystallized from methylene chloride/hexane.
A warm solution of 6.5 g (0.02 mol) of 8-chloro-6-(2-fluorophenyl)-1-methyl-
4H-imidazo[1,5-a] [1,4]-benzodiazepine in 30 ml of ethanol was combined
with a warm solution of 2.6 g (0.022 mol) of maleic acid in 20 ml of ethanol.
The mixture was diluted with 150 ml of ether and heated on the steam bath
for 3 minutes. After cooling, the crystals were collected, washed with ether
and dried in vacuo to yield 8-chloro-6-(2-fluorophenyl)-1-methyl-4H�imidazo[1.5-a] [1,4]-benzodiazepine maleate, melting point 148°C to 151°C. | [Therapeutic Function]
Anesthetic | [Biochem/physiol Actions]
Sedative/Hypnotic; ligand for the GABAA receptor benzodiazepine modulatory site; CYP3A4 substrate. | [Synthesis]
The preparation starts with 7-chloro-
5-(2-fluorophenyl)?1,3-dihydro-2H-1,4-
benzodiaze-pinone . For the literature, also
see .
Midazolam is approximately twice as active
as diazepam, causes less pain at the injection
site, and has a shorter half-life than diazepam.
Side effects: dose-dependent cerebral depression
with tranquilization, sedation, and dryness.
Reduction in blood pressure, respiratory depression,
and cardiovascular effects were slight.
 |
|