Ozenoxacin
|
|
|
- CAS-Nr.
- 245765-41-7
- Englisch Name:
- Ozenoxacin
- Synonyma:
- T 3912;M-5120;Ozenoxacin;Ozefloxacin;GF-00100100;GF-001001-00;Ozenoxacin, >Ozenoxacin, >90%;Ozenoxacin(T 3912);Ozenoxacin USP/EP/BP
- CBNumber:
- CB62498834
- Summenformel:
- C21H21N3O3
- Molgewicht:
- 363.41
- MOL-Datei:
- 245765-41-7.mol
|
Ozenoxacin Eigenschaften
- Schmelzpunkt:
- >255°C (dec.)
- Siedepunkt:
- 573.5±50.0 °C(Predicted)
- Dichte
- 1.372±0.06 g/cm3(Predicted)
- storage temp.
- 2-8°C(protect from light)
- Löslichkeit
- DMSO (Slightly)
- pka
- 6.46±0.50(Predicted)
- Aggregatzustand
- Solid
- Farbe
- Off-White
Sicherheit
- Risiko- und Sicherheitserklärung
- Gefahreninformationscode (GHS)
| Bildanzeige (GHS) |

|
| Alarmwort |
Warnung |
| Gefahrenhinweise |
| Code |
Gefahrenhinweise |
Gefahrenklasse |
Abteilung |
Alarmwort |
Symbol |
P-Code |
| H302 |
Gesundheitsschädlich bei Verschlucken. |
Akute Toxizität oral |
Kategorie 4 |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
P264, P270, P301+P312, P330, P501 |
| H315 |
Verursacht Hautreizungen. |
Hautreizung |
Kategorie 2 |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
P264, P280, P302+P352, P321,P332+P313, P362 |
| H319 |
Verursacht schwere Augenreizung. |
Schwere Augenreizung |
Kategorie 2 |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
P264, P280, P305+P351+P338,P337+P313P |
| H335 |
Kann die Atemwege reizen. |
Spezifische Zielorgan-Toxizität (einmalige Exposition) |
Kategorie 3 (Atemwegsreizung) |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
|
|
| Sicherheit |
| P261 |
Einatmen von Staub vermeiden. |
| P280 |
Schutzhandschuhe/Schutzkleidung/Augenschutz tragen. |
| P301+P312 |
BEI VERSCHLUCKEN: Bei Unwohlsein GIFTINFORMATIONSZENTRUM/Arzt/... (geeignete Stelle für medizinische Notfallversorgung vom Hersteller/Lieferanten anzugeben) anrufen. |
| P302+P352 |
BEI BERÜHRUNG MIT DER HAUT: Mit viel Wasser/... (Hersteller kann, falls zweckmäßig, ein Reinigungsmittel angeben oder, wenn Wasser eindeutig ungeeignet ist, ein alternatives Mittel empfehlen) waschen. |
| P305+P351+P338 |
BEI KONTAKT MIT DEN AUGEN: Einige Minuten lang behutsam mit Wasser spülen. Eventuell vorhandene Kontaktlinsen nach Möglichkeit entfernen. Weiter spülen. |
|
Ozenoxacin Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Ozenoxacin is a novel,
nonfluorinated quinolone antibiotic discovered by Toyama
Chemical Co. Ltd. and developed by Maruho Co. Ltd.
Ozenoxacin was approved by the PMDA of Japan in September
2015 for the treatment of acne and skin infections. Ozenoxacin
shows potent antibacterial activity against anaerobic and
aerobic, gram-positive and -negative bacteria, especially those implicated in superficial skin infections such as S. aureus,
Staphylococcus epidermidis, and Propionibacterium acnes. The
mechanism of action of ozenoxacin involves the drug’s affinity
for DNA gyrase and DNA topoisomerase IV and upon binding
triggers bacterial apoptosis.
Verwenden
Ozenoxacin is a non-fluorinated topical quinolone. It exhibits antimicrobial activity against?propionibacteria and staphylococci. Ozenoxacin can be used to treat acne and superficial skin infections.
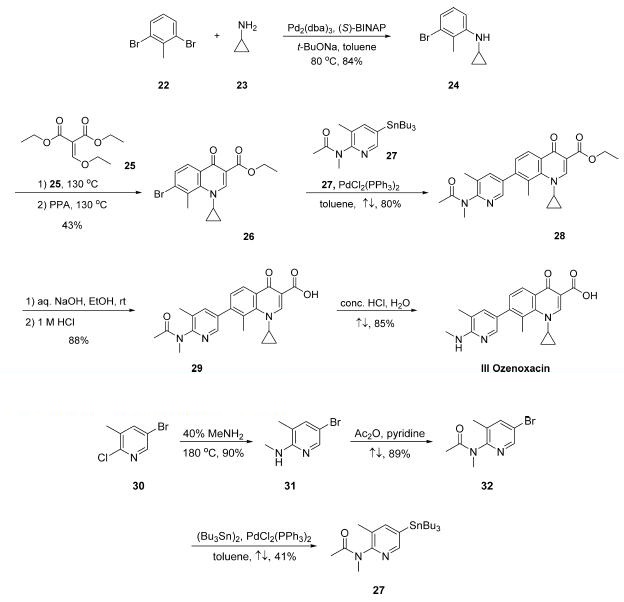
Synthese
A U.S. patent filed by co-workers at Toyama describes the
only publicly disclosed synthetic approach to this drug.12 The
drug?ˉs assembly hinges upon a key Stille coupling between a
quinolonyl bromide and a stannylpyridine.
Buchwald-Hartwig coupling of commercially available 2,6-
dibromotoluene (22) and cyclopropylamine (23) gave Ncyclopropyl-
3-bromo-2-methylaniline 24 in 84% yield , and this step was followed by reaction with diethyl
ethoxymethylenemalonate (25) and subsequent cyclization
under acidic conditions to secure bromoquinoline 26 in 43%
yield over the two-step sequence. Stille coupling of 27 with
bromoquinoline 26 resulted in pyridyl quinoline adduct 28 in
80% yield. Saponification of ester 28 followed by acidic removal
of the N-acetyl group delivered the active pharmaceutical
ingredient ozenoxacin (III) in 75% yield.
The preparation of key stannane 27, which is not
commercially available and began
with the conversion of commercially available 5-bromo-2-
chloro-3-methylpyridine (30) to aminopyridine derivative 31
upon treatment with aqueous methylamine at elevated
temperature in a sealed vessel. The resulting aminopyridine
was subjected to acetic anhydride in pyridine, resulting in
acetamide 32 in good yield, and this coupling was followed by a
modest-yielding palladium-catalyzed installation of the stannyl
group to deliver subunit 27.
Ozenoxacin Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Ozenoxacin Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global( 179)Lieferanten
- Ozenoxacin
- T 3912
- :3-Quinolinecarboxylic acid, 1-cyclopropyl-1,4-dihydro-8-methyl-7-[5-methyl-6-(methylamino)-3-pyridinyl]-4-oxo-
- Ozenoxacin(T 3912)
- 1-cyclopropyl-8-methyl-7-[5-methyl-6-(methylamino)pyridin-3-yl]-4-oxoquinoline-3-carboxylic acid
- Ozenoxacin , T 3912,UNII-V0LH498RFO
- Ozenoxacin Impurity 1
- organic materials Ozenoxacin
- Ozenoxacin, >
- 1-cyclopropyl-8-methyl-7-(5-methyl-6-(methylamino)pyridin-3-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
- Ozenoxacin USP/EP/BP
- Ozenoxacin, >90%
- Ozefloxacin
- GF-00100100
- GF-001001-00
- M-5120
- 245765-41-7

