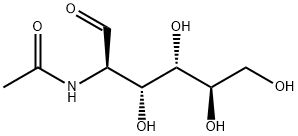
N-Acetylglucosamine
- CAS No.
- 7512-17-6
- Chemical Name:
- N-Acetylglucosamine
- Synonyms
- N-ACETYLGLUCOSAMINE;D-GLCNAC;acetylglucosamine;2-Acetamido-2-deoxy-D-glucopyranose;Acetyl chitin;N-Acetyl-D-glucosamin;N-ACETYL-D-GLUCOSAMINIDE;N-Acetyl-beta-D-glucosamine;2-ACETAMIDO-2-DEOXY-D-GLUCOSE;NADG
- CBNumber:
- CB00125841
- Molecular Formula:
- C8H15NO6
- Molecular Weight:
- 221.21
- MDL Number:
- MFCD00136044
- MOL File:
- 7512-17-6.mol
- MSDS File:
- SDS
| Melting point | 211 °C (dec.) (lit.) |
|---|---|
| alpha | 42 º (c=2,water,2 hrs) |
| vapor pressure | 8Pa at 20℃ |
| refractive index | +39.0 to +42.0 ° (C=1, H2O) |
| storage temp. | -20°C |
| solubility | Soluble in concentrated hydrochloric acid, sulfuric acid, phosphoric acid and formic acid. Insoluble in water, dilute acids, dilute alkalis, concentrated alkalis and organic solvents. |
| form | saline suspension |
| Boiling point | 636.4±55.0 °C(Predicted) |
| Density | 1.54 g/cm3 |
| pka | 13.04±0.20(Predicted) |
| color | white to off-white |
| optical activity | Alpha type +75 → +41 Beta type -22 → +41.3 |
| biological source | crab (red) |
| Water Solubility | H2O: 50 mg/mL colorless to faint yellow solution, clear to very slightly hazy |
| Sensitive | Hygroscopic |
| Merck | 14,4458 |
| BRN | 1727589 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | OVRNDRQMDRJTHS-ROGOILFBSA-N |
| LogP | -2.2 at 23.7℃ |
| CAS DataBase Reference | 7512-17-6(CAS DataBase Reference) |
| FDA UNII | V956696549 |
| EPA Substance Registry System | N-Acetylglucosamine (7512-17-6) |
| UNSPSC Code | 41116107 |
| NACRES | NA.75 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314 | |||||||||
| Precautionary statements | P280-P305+P351+P338-P310 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 24/25-36-26-22 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | LZ6651000 | |||||||||
| F | 3-10 | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29329985 | |||||||||
| NFPA 704 |
|
N-Acetylglucosamine price More Price(79)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A3286 | N-Acetyl-D-glucosamine suitable for cell culture, BioReagent | 7512-17-6 | 5g | $36.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1010022 | N-Acetyl-D-glucosamine United States Pharmacopeia (USP) Reference Standard | 7512-17-6 | 200mg | $383 | 2024-03-01 | Buy |
| TCI Chemical | A0092 | N-Acetyl-D-glucosamine >98.0%(HPLC)(N) | 7512-17-6 | 25g | $37 | 2024-03-01 | Buy |
| TCI Chemical | A0092 | N-Acetyl-D-glucosamine >98.0%(HPLC)(N) | 7512-17-6 | 100g | $106 | 2024-03-01 | Buy |
| Alfa Aesar | A13047 | N-Acetyl-D-glucosamine, 98+% | 7512-17-6 | 10g | $28.8 | 2023-06-20 | Buy |
N-Acetylglucosamine Chemical Properties,Uses,Production
Cell Structure Components
N-Acetylglucosamine (GlcNAc) is a key component of bacterial cell wall peptidoglycan, fungal cell wall chitin, and animal cell extracellular matrix, and plays an important structural role on the cell surface. It has been shown that GlcNAc has a novel role in cell signalling that stimulates morphological changes and virulence gene expression in the human fungal pathogen Candida albicans. Pathogenic Escherichia coli responds to GlcNAc by altering the expression of cilia and CURLI fibres that promote biofilm formation. Studies in animal cells have shown that GlcNAc affects cell signalling by post-translational modification of proteins through glycosylation.The O-bonding of GlcNAc to Ser and Thr residues regulates a wide range of intracellular proteins, including transcription factors such as NFκB, c-myc and p53. In addition, the specificity of the Notch family of receptors for different ligands is altered by GlcNAc attachment to fucose residues in the extracellular structural domain.GlcNAc also affects cell surface signalling proteins by altering the degree of branching of N-linked glycans that influence signal transduction.
Uses
N-Acetylglucosamine (GlcNAc) is widely used as a nutritional supplement to aid joint health and treat osteoarthritis. In addition, GlcNAc also plays a key role in cell signalling, similar to other common types of messenger molecules, including ions, nucleotides and amino acids. Studies have shown GlcNAc to have beneficial therapeutic effects in patients with inflammatory bowel disease.
Mechanism of action
The mechanism of action in relieving arthritic pain and in repair of cartilage is a matter of speculation. Biochemically, glucosamine is involved in glycoprotein metabolism. Glycoproteins, known as proteoglycans, form the ground substance in the extra-cellular matrix of connective tissue. Proteoglycans are polyanionic substances of high-molecular weight and contain many different types of heteropolysaccharide side-chains covalently linked to a polypeptide-chain backbone. These polysaccharides make up to 95% of the proteoglycan structure. In fact, chemically, proteoglycans resemble polysaccharides more than they do proteins. The polysaccharide groups in proteoglycans are called glycosaminoglycans (GAGs). GAGs include hyaluronic acid, chondroitin sulfate, dermatan sulfate, keratan sulfate, heparin and heparan sulfate. All of the GAGs contain derivatives of glucosamine or galactosamine. Glucosamine derivatives are found in hyaluronic acid, keratan sulfate and heparan sulfate. Chondroitin sulfate contains derivatives of galactosamine. The glucosamine-containing glycosaminoglycan hyaluronic acid is vital for the function of articular cartilage. GAG chains are fundamental components of aggrecan found in articular cartilage. Aggrecan confers upon articular cartilage shock-absorbing properties. It does this by providing cartilage with a swelling pressure that is restrained by the tensile forces of collagen fibers. This balance confers upon articular cartilage the deformable resilience vital to its function. In the early stages of degenerative joint disease, aggrecan biosynthesis is increased. However, in later stages, aggrecan synthesis is decreased, leading eventually to the loss of cartilage resiliency and to most of the symptoms that accompany osteoarthritis. During the progression of osteoarthritis, exogenous glucosamine may have a beneficial role. It is known that, in vitro, chondrocytes do synthesize more aggregan when the culture medium is supplemented with glucosamine. N-acetylglucosamine is found to be less effective in these in vitro studies. Glucosamine has also been found to have antioxidant activity and to be beneficial in animal models of experimental arthritis. The counter anion of the glucosamine salt (i.e. chloride or sulfate) is unlikely to play any role in the action or pharmacokinetics of glucosamine. Further, the sulfate in glucosamine sulfate supplements should not be confused with the glucosamine sulfate found in such GAGs as keratan sulfate and heparan sulfate. In the case of the supplement, sulfate is the anion of the salt. In the case of the above GAGs, sulfate is present as an ester. Also, there is no glucosamine sulfate in chondroitin sulfate (source: PDRhealth).
N-Acetylglucosamine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Huzhou Chenxi Environmental Protection Technology Co. , Ltd. | +8616762203950 | 1034385273@qq.com | China | 55 | 58 |
| Watson Biotechnology Co.,Ltd | +86-18186686046 +86-18186686046 | sales01@watsonbiotech.cn | China | 5849 | 58 |
| Henan Alfa Chemical Co., Ltd | China | 12341 | 58 | ||
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8804 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | 1022@dideu.com | China | 3882 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5887 | 58 |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-17333973358 | sales06@hbduling.cn | China | 8051 | 58 |
| Anhui Yiao New Material Technology Co., Ltd | +86-18033737140 +86-17354101231 | sales1@hbganmiao.com | China | 227 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Wuhan Haorong Biotechnology Co.,ltd | +86-18565342920; +8618565342920 | sales@chembj.net | China | 289 | 58 |
Related articles
- N-Acetyl-D-glucosamine: used for the treatment of Osteoarthritis
- N-Acetyl-D-glucosamine, or glcnac or N-acetylchitosamine, belongs to the class of organic compounds known as acylaminosugars.
- Jul 25,2024
- Functions of N-acetyl glucosamine
- N-acetyl glucosamine (NAG) is a newer pigment reduction agent. It is an amino-monosaccharide produced in the body by adding an....
- Mar 4,2022
View Lastest Price from N-Acetylglucosamine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-02-12 | N-acetylgalactosaminyl-1-4-N-acetylglucosamine
136198-41-9
|
US $0.00 / G | 10G | 98%min | 30kg/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-12-24 | acetylglucosamine
7512-17-6
|
US $0.00-0.00 / Kg | 1Kg | 99.9% | 200tons | airuikechemical co., ltd. | |
 |
2024-05-09 | N-Acetyl-D-Glucosamine;N-Acetylglucosamine
7512-17-6
|
US $0.00 / kg | 1kg | 99% | 10000kg | Shaanxi TNJONE Pharmaceutical Co., Ltd |
-

- N-acetylgalactosaminyl-1-4-N-acetylglucosamine
136198-41-9
- US $0.00 / G
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- acetylglucosamine
7512-17-6
- US $0.00-0.00 / Kg
- 99.9%
- airuikechemical co., ltd.
-

- N-Acetyl-D-Glucosamine;N-Acetylglucosamine
7512-17-6
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd