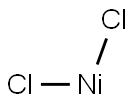Nickel chloride
- CAS No.
- 7718-54-9
- Chemical Name:
- Nickel chloride
- Synonyms
- NiCl2;NiCl;NICKEL DICHLORIDE;NICKEL(II) CHLORIDE;Hexahydrate;LiCl2.6H2O;dichloronickel;NICKEL CHLORIDE;Nichel chloride;NICKEL(+2)CHLORIDE
- CBNumber:
- CB0116468
- Molecular Formula:
- Cl2Ni
Lewis structure

- Molecular Weight:
- 129.6
- MDL Number:
- MFCD00011142
- MOL File:
- 7718-54-9.mol
- MSDS File:
- SDS
| Melting point | 1001 °C | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boiling point | 987°C | ||||||||||||||
| Density | 3.55 g/mL at 25 °C(lit.) | ||||||||||||||
| vapor pressure | 1.33 hPa (671 °C) | ||||||||||||||
| storage temp. | Store below +30°C. | ||||||||||||||
| solubility | H2O: soluble | ||||||||||||||
| form | powder | ||||||||||||||
| Specific Gravity | 3.55 | ||||||||||||||
| color | Yellow to orange | ||||||||||||||
| PH | 4 (500g/l, H2O, 20℃) | ||||||||||||||
| Water Solubility | slightly soluble | ||||||||||||||
| Sensitive | Hygroscopic | ||||||||||||||
| Crystal Structure | CdCl2 type | ||||||||||||||
| crystal system | Three sides | ||||||||||||||
| Merck | 14,6505 | ||||||||||||||
| Space group | R3m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits |
ACGIH: TWA 0.1 mg/m3 NIOSH: IDLH 10 mg/m3; TWA 0.015 mg/m3 |
||||||||||||||
| Stability | Stable. Incompatible with peroxides. | ||||||||||||||
| InChIKey | QMMRZOWCJAIUJA-UHFFFAOYSA-L | ||||||||||||||
| CAS DataBase Reference | 7718-54-9(CAS DataBase Reference) | ||||||||||||||
| EWG's Food Scores | 1 | ||||||||||||||
| FDA UNII | 696BNE976J | ||||||||||||||
| NIST Chemistry Reference | Nickel dichloride(7718-54-9) | ||||||||||||||
| EPA Substance Registry System | Nickel(II) chloride (7718-54-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H331-H315-H317-H334-H341-H350i-H360D-H372-H410 | |||||||||
| Precautionary statements | P201-P273-P280-P301+P310-P302+P352-P304+P340+P311 | |||||||||
| Hazard Codes | T,N,Xi | |||||||||
| Risk Statements | 45-25-36/38-43-50-50/53-22-52/53-68-51/53-48/23-42/43-20/22-61-49-38-23/25 | |||||||||
| Safety Statements | 53-26-36/37-45-61-37-29-24-23-60 | |||||||||
| RIDADR | UN 3288 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | QR6475000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28273500 | |||||||||
| Toxicity | LD50 in mice, rats (mg/kg): 48, 11 i.p. (IARC) | |||||||||
| NFPA 704 |
|
Nickel chloride price More Price(48)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.06722 | Nickel(II) chloride anhydrous for synthesis | 7718-54-9 | 100G | $134 | 2024-03-01 | Buy |
| Sigma-Aldrich | 339350 | Nickel(II) chloride 98% | 7718-54-9 | 50g | $63 | 2024-03-01 | Buy |
| Sigma-Aldrich | 339350 | Nickel(II) chloride 98% | 7718-54-9 | 250g | $242 | 2024-03-01 | Buy |
| TCI Chemical | N0850 | Nickel(II) Chloride Anhydrous >98.0%(T) | 7718-54-9 | 25g | $57 | 2024-03-01 | Buy |
| TCI Chemical | N0850 | Nickel(II) Chloride Anhydrous >98.0%(T) | 7718-54-9 | 500g | $295 | 2024-03-01 | Buy |
Nickel chloride Chemical Properties,Uses,Production
Description
Nickel chloride is a brown or green colored solid. Most nickel chloride is used for electroplating and nickel plating, used to prepare various nickel salts and nickel catalysts; . During electroplating, it accelerates the dissolution of anode metal nickel, while acting to increase the electrolyte level of the solution. Nickel chloride can be used as catalyst for organic synthesis (e.g. pyrazolophthalazinyl spirooxindoles), to synthesize nickel nanoparticles as a reductant, and to prepare nickel coordination compounds, and and in industrial gas masks to protect from ammonia. It can also be used as an NH3 absorbent in gas masks and as a source of nickel ion for cell culture and toxicological studies.
Properties
NICKEL CHLORIDE is a brown or green colored solid. Its hexahydrate is a green monoclinic crystal, which is weathered in dry air and deliquescent in moist air. It is easily soluble in water, ethanol and ammonia. It is easily reduced to nickel when heated in hydrogen and becomes nickel oxide by heating in the air. It loses water of crystallization and becomes anhydrous in high temperature. The aqueous solution is acidic. Nickel chloride can combine with the anions of many inorganic and organic molecules to form nickel complexes, and form insoluble precipitates in weak acids.
Preparation
Anhydrous nickel chloride is prepared by burning nickel in chlorine gas.
Some other methods of preparation involve
(1) the action of acetyl chloride on nickel acetate in a nonaqueous solvent such as benzene:
(CH3COO)2Ni + 2CH3COCl → NiCl2 + 2CH3COOCOCH3
(2) the action of thionyl chloride on nickel chloride hexahydrate:
NiCl2•6H2O + 6SOCl2 → NiCl2 + 12HCl + 6SO2
(3) heating nickel chloride hexahydrate or nickel chloride ammoniate:
NiCl2•6H2O → NiCl2 + 6H2O
NiCl2•6NH3 → NiCl2 + 6NH3
The hexahydrate is prepared either by the action of hot dilute hydrochloric acid on nickel powder or by dissolving nickel oxide in dilute hydrochloric acid followed by crystallization. For the preparation of ammoniate, see Reactions below.
Reaction
When ammonia gas is passed over anhydrous nickel chloride the product is an ammoniate, hexamine nickel chloride, NiCl2•6NH3. Ammoniate also can be prepared in solution by dissolving nickel chloride hexahydrate in an aqueous solution of ammonia.
Nickel chloride forms double salts with alkali metal chlorides or ammonium chloride. Such double salts, NH4Cl•NiCl2•6H2O, are obtained as hexahydrate when crystallized from a mixed solution of nickel chloride and ammonium chloride in equimolar amounts.
Warming a solution of nickel chloride and sodium hydroxide at moderate concentrations may partially precipitate a basic salt of indefinite composition. The average composition of this salt is NiCl2•3Ni(OH)2. Salt composition may vary depending on reaction conditions.
When hydrogen sulfide is passed through a buffered solution of nickel chloride, nickel sulfide, NiS, precipitates.
An alcoholic solution of nickel chloride, when treated with an ethereal solution of dithiobenzoic acid, C6H5CSSH, blue nickel(II) dithiobenzoate,(C6H5CSS)2Ni, is formed:
NiCl2 + 2C6H5CSSH→ Ni (C6H5CSS)2 + 2HCl
The product oxidizes readily to a violet dimeric nickel(IV) complex.
Health Hazards
Acute Health Effects
The following acute (short-term) health effects may occur immediately or shortly after exposure to Nickel Chloride:
- Contact can irritate and bum the skin and eyes.
- Breathing Nickel Chloride can irritate the nose, throat and lungs causing cough, phlegm and shortness of breath.
The following chronic (long-term) health effects can occur at some time after exposure to Nickel Chloride and can last for months or years:
Cancer Hazard
- Nickel Chloride may cause mutations (genetic changes). Whether or not it poses a cancer or reproductive hazard needs further study.
- There is limited evidence that Nickel Chloride is a teratogen in animals. Until further testing has been done, it should be treated as a possible teratogen in humans.
- Nickel Chloride may cause a skin allergy. If allergy develops, very low future exposure can cause itching and a skin rash.
- Nickel Chloride may cause an asthma-like allergy. Future exposure can cause asthma attacks with shortness of breath, wheezing, cough, and/or chest tightness.
- Repeated exposure may cause scarring of the lungs and may affect the kidneys.
References
[1] X. N. Zhang, Y. X. Li and Z. H. Zhang, Nickel chloride-catalyzed one-pot three-component synthesis of pyrazolophthalazinyl spirooxindoles, Tetrahedron, 2011, vol. 67, 7426-7430
[2] Z. Jiang, J. Xie, D. Jiang, X. Wie and M. Chen, Modifiers- assisted formation of nickel nanoparticles and their catalytic application to p-nitrophenol reduction, CrystEngComm, 2013, vol. 15, 560-569
[3] NA Eckert, EM Bones, RJ Lachicotte and PL Holland, Nickel complexes of a bulky beta-diketiminate ligand, Inorg. Chem., 2003, vol. 42, 1720-1725
Description
Nickel chloride, NiCl2, is a water-soluble salt used in a variety of industries. It is typically produced and used either as an anhydrous salt or as a hydrate: nickel chloride hexahydrate as well as nickel chloride dihydrate. Nickel chloride is very rarely found in nature, and is produced via extraction from nickelcontaining ores via treatment with hydrochloric acid.
Chemical Properties
Brown scales, deliquescent
Chemical Properties
Nickel chloride appears as green or brown scales, or sparkling golden-yellow powder.
Physical properties
The anhydrous salt forms yellow crystal scales; deliquesces; density 3.55 g/cm3; melts at 1,001°C; sublimes at 973°C; highly soluble in water, 64 g/100mL at 20°C; soluble in alcohol.
The hexahydrate forms green monoclinic crystals; deliquesces; extremely soluble in water, 254 g/100mL at 20°C, and about 600 g/100 ml at 100°C; also very soluble in alcohol.
Uses
Nickel chloride is used for nickel plating cast zinc, as an agent in electrolytic refining of nickel, as a chemical intermediate for nickel catalysts and complex nickel salts, as an absorber of ammonia gas in industrial gas masks, as a catalyst in diarylamine and silicon tetrachloride production, as an agent in electrodeless plating of nickel, as an agent in tin–nickel alloy plating, and as a fungicide for control of rust and rustlike disease. However, workers exposed to different forms of nickel have an elevated risk of lung cancer. Besides, Ni and its compounds (particularly insoluble compounds of nickel) have been reported to be potent carcinogens and toxic agents in humans and experimental animals. Therefore, Ni compounds are considered to be an industrial/occupational health hazard.
Uses
Nickel chloride (NiCl2) is used for electroplating nickel onto the surfaces of other metals and as a chemical reagent in laboratories.
Uses
Nickel chloride solutions are used for electroplating nickel onto other metal items.It also is used to prepare various nickel salts and nickel catalysts; and in industrial gas masks to protect from ammonia.
Definition
ChEBI: A compound of nickel and chloride in which the ratio of nickel (in the +2 oxidation state) to chloride is 1:2.
Nickel chloride is a yellow deliquescent solid with a boiling point of 973°C(1690°F). Nickel chloride is soluble in water and alcohol. Nickel chloride(hydrated),NiCI2·H20, is a gray deliquescent solid that is also soluble in water and alcohol.It is used in nickel plating.
Definition
hexahydrate: A crystalline compoundthat has six moles of waterper mole of compound.
Production Methods
Nickel chloride (hexahydrate) is obtained by reacting metal nickel powder or nickel oxide with hot, dilute hydrochloric acid.
Preparation
Nickel(II) chloride can be obtained by reaction of the elements either in a flow system at high temperatures or by reaction in ethanol at 20°. It is readily prepared in the laboratory by dehydration of the hexahydrate with thionyl chloride.
General Description
Nickel(II) chloride (NiCl2) is a nickel based halide that is prepared by burning nickel in chlorine. It is a water soluble compound that crystallizes to form a hexahydrate. It is majorly utilized in organic synthesis as a catalyst and a precursor.
Hazard
Confirmed carcinogen.
Flammability and Explosibility
Non flammable
Safety Profile
Confirmed human carcinogen. Poison by ingestion, intravenous, intramuscular, and intraperitoneal routes. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits very toxic fumes of Cl-. See also NICICEL COMPOUNDS.
Potential Exposure
Nickel chloride is used in electroplating and ink manufacturing.
Environmental Fate
Nickel chloride is water soluble (642 g l-1 for anhydrous;
2540 g l-1 for hexahydrate) and would be expected to release
divalent nickel into the water. Since nickel chloride quickly
dissolves upon exposure to moist environments, and partially
due to the ubiquity of nickel in soil, water, and air, tracking the
course of the salt through the environment is difficult. This is
particularly due to nickel’s ability to complex with anionic
species other than chloride to form nickel oxide, sulfate, nitrate,
carbonate, or acetate, among others.
Industrial uses of nickel chloride result in nickel being
distributed mainly at soil surfaces and through surrounding
waterways and water tables. Once distributed to the soil, nickel
chloride produces nickel(II) ions to potentially form inorganic
crystalline minerals or precipitates, can complex or adsorb onto
organic and inorganic surfaces, can participate in cation
exchange, and can exist as free-ion or chelated metal complexes
in soil solution.
Shipping
UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
It crystallises from dilute HCl to form the green hexahydrate. At 70o this dehydrates to the tetrahydrate, and at higher temperatures it forms the anhydrous salt. It sublimes in yellow hexagonal scales in a stream of HCl. Store it in a desiccator as it is deliquescent. [Hart & Partington J Chem Soc 104 1943.]
Toxicity evaluation
The parent metal alters sodium balance and lipid metabolism; it induces metallothionein synthesis. Nickel chloride affects the T-cell portion of the immune system and suppresses the activity of natural killer cells. If given orally or by inhalation, nickel chloride has been reported to decrease iodine uptake by the thyroid gland. The lipid peroxidation properties of nickel can introduce potential malignancies in humans, as DNA strand gaps and breaks in DNA–protein cross-links can form. The downregulation of glycoprotein metabolism by nickel ions may produce nephrotoxicity in humans as well.
Incompatibilities
Strong acids, potassium, sulfur. Forms an impact-sensitive mixture with potassium.
Waste Disposal
Recycle or disposal in a chemical waste landfill is recommended.
Nickel chloride Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29811 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9636 | 58 |
View Lastest Price from Nickel chloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-10-25 | NiCl2
7718-54-9
|
US $1.00 / kg | 1kg | Ni:42%min | 20tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-09-23 | Nickel chloride
7718-54-9
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-05-04 | nickel chloride
7718-54-9
|
US $48.00-40.00 / KG | 1KG | 99% | 200tons | Guangzhou Sunton Biotechnology Co., Ltd. |
-

- NiCl2
7718-54-9
- US $1.00 / kg
- Ni:42%min
- Hebei Yanxi Chemical Co., Ltd.
-

- Nickel chloride
7718-54-9
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- nickel chloride
7718-54-9
- US $48.00-40.00 / KG
- 99%
- Guangzhou Sunton Biotechnology Co., Ltd.