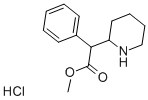
Methylphenidate hydrochloride
- CAS No.
- 298-59-9
- Chemical Name:
- Methylphenidate hydrochloride
- Synonyms
- METHYLPHENIDATE HCL;Concerta;methylphenidate hydrochloride methanol*solution;Equasym;Metadate;Centedin;Centedrin;Centedrine;NSC 169868;Ciba 4311b
- CBNumber:
- CB0188559
- Molecular Formula:
- C14H20ClNO2
- Molecular Weight:
- 269.77
- MDL Number:
- MFCD00058191
- MOL File:
- 298-59-9.mol
- MSDS File:
- SDS
| Melting point | 196-198?C |
|---|---|
| Flash point | 9℃ |
| storage temp. | Store at RT |
| solubility | Freely soluble in water, soluble in ethanol (96 per cent), slightly soluble in methylene chloride. |
| form | Powder |
| pka | 8.9(at 25℃) |
| Water Solubility | Soluble to 50 mM in water and to 100 mM in ethanol |
| BCS Class | 2 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, alkalies, barbiturates. |
| InChI | InChI=1S/C14H19NO2.ClH/c1-17-14(16)13(11-7-3-2-4-8-11)12-9-5-6-10-15-12;/h2-4,7-8,12-13,15H,5-6,9-10H2,1H3;1H |
| InChIKey | JUMYIBMBTDDLNG-UHFFFAOYSA-N |
| SMILES | C(C(=O)OC)(C1NCCCC1)C1C=CC=CC=1.Cl |
| CAS DataBase Reference | 298-59-9(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | Concerta; methylphenidate hydrochloride |
| FDA UNII | 4B3SC438HI |
| NCI Drug Dictionary | Concerta |
| EPA Substance Registry System | Methylphenidate hydrochloride (298-59-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H302-H334 |
| Precautionary statements | P261-P264-P270-P284-P301+P312-P501 |
| Hazard Codes | Xn,T,F |
| Risk Statements | 22-42-39/23/24/25-23/24/25-11 |
| Safety Statements | 22-26-36-45-36/37-16 |
| RIDADR | 3249 |
| WGK Germany | 3 |
| RTECS | TM3850000 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| Toxicity | LD50 orally in mice: 190 mg/kg (Greenblatt, Osterberg) |
Methylphenidate hydrochloride Chemical Properties,Uses,Production
Chemical Properties
white crystalline powder
Originator
Ritalin,Ciba,US,1958
Uses
Methylphenidate (a.k.a., Ritalin) is a schedule II drug in the United States commonly used as a psychostimulant for the treatment of attention-deficit hyperactivity disorder. It blocks dopamine and norepinephrine transporters as well as facilitates NMDA-receptor mediated synaptic transmission through σ1 receptors via PLC/PKC signaling. This interaction with the σ1 receptor has been suggested to underlie methylphenidate’s considerable abuse potential and potential psychiatric side effects. This product is intended for forensic and biological research applications.[Cayman Chemical]
Uses
antiinflammatory
Uses
Controlled substance (stimulant). CNS stimulant. Four isomers of Methylphenidate are known to exist. One pair of threo isomers and one pair of erythro are distinguished, from which only d-threo-Methyl phenidate exhibits the pharmacologically usually desired effects.
Manufacturing Process
As described in US Patent 2,507,631, 80 g of pulverized sodium amide are gradually added, while stirring and cooling, to a solution of 117 g of phenylacetonitrile and 113 g of 2-chloropyridine in 400 cc of absolute toluene. The mixture is then slowly heated to 110° to 120°C and maintained at this temperature for 1 hour. Water is added thereto after cooling, the toluene solution is shaken with dilute hydrochloric acid and the hydrochloric acid extracts are made alkaline with concentrated caustic soda solution. A solid mass is separated thereby which is taken up in acetic ester and distilled, αphenyl-α-pyridyl-(2)-acetonitrile passing over at 150° to 155°C under 0.5 mm pressure. When recrystallized from ethyl acetate it melts at 88° to 89°C, the
yield amounting to 135 g.
100 g of α-phenyl-α-pyridyl-(2)-acetonitrile are introduced into 400 cc of
concentrated sulfuric acid, allowed to stand overnight at room temperature,
poured into ice and rendered alkaline with sodium carbonate. α-Phenyl-αpyridyl-(2)-acetamide is precipitated thereby which melts at 134°C after
recrystallization from ethyl acetate.
100g of the resulting α-phenyl-α-pyridyl-(2)-acetamide, when dissolved in one
liter of methyl alcohol and treated for 6 hours at water-bath temperature with
hydrogen chloride, and after concentrating, diluting with water and rendering
alkaline with sodium carbonate, yield 90 g of the α-phenyl-α-pyridyl-(2)-acetic
acid methylester of MP 74° to 75°C (from alcohol of 50% strength).
The α-phenyl-α-piperidyl-(2)-acetic acid methylester of BP 135° to 137°C
under 0.6 mm pressure is obtained in theoretical yield by hydrogenation of 50
g of α-phenyl-α-pyridyl(2)-acetic acid methylester in glacial acetic acid in the
presence of 1 g of platinum catalyst at room temperature, while taking up 6
hydrogen atoms. Reaction with HCl gives the hydrochloride. Resolution of
stereoisomers is described in US Patent 2,957,880.
Therapeutic Function
Psychostimulant
General Description
Because methylphenidate (Ritalin) has two asymmetriccenters, there are four possible isomers. The threo racemateis the marketed compound and is about 400 times aspotent as the erythro racemate.The absolute configurationof each of the threo-methylphenidate isomers has beendetermined.Considering that the structure is fairly complex(relative to amphetamine), it is likely that one of thetwo components of the threo racemate contains most of theactivity. Evidence indicates that the (+) -(2R,2'R)threoisomer is involved principally in the behavioral andpressor effects of the racemate.As is likely with many central psychomotor stimulants, there are multiple modesof action.
Methylphenidate, probably largely via its p-hydroxymetabolite, blocks NE reuptake, acts as a postsynapticagonist, depletes the same NE pools as reserpine, and haseffects on dopaminergic systems, such as blocking DAreuptake.
Methylphenidate is a potent CNS stimulant. Indicationsinclude narcolepsy and attention-deficit disorder.
General Description
Odorless white crystalline powder. Metallic taste. Solutions are acid to litmus. Absence of general acid/base catalysis.
Air & Water Reactions
Water soluble.
Reactivity Profile
Methylphenidate hydrochloride is incompatible with alkalis and solutions of barbiturates . Undergoes typical ester hydrolysis in aqueous solutions at 176°F.
Fire Hazard
Flash point data for Methylphenidate hydrochloride are not available; however, Methylphenidate hydrochloride is probably combustible.
Biological Activity
Psychomotor stimulant. Inhibitor of dopamine and noradrenalin transporters that increases the extracellular concentration of dopamine and noradrenalin. Increases locomotor activity in vivo .
Veterinary Drugs and Treatments
Methylphenidate may be useful for treating cataplexy/narcolepsy or hyperactivity in dogs.
storage
Store at RT
Methylphenidate hydrochloride Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
298-59-9(Methylphenidate hydrochloride)Related Search:
1of4