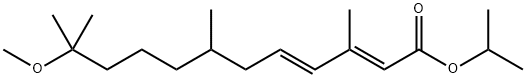
METHOPRENE
- CAS No.
- 40596-69-8
- Chemical Name:
- METHOPRENE
- Synonyms
- apex;kabat;manta;minex;ZR-515;PRECOR;diacon;dianex;altosid;Apex 5E
- CBNumber:
- CB0429636
- Molecular Formula:
- C19H34O3
- Molecular Weight:
- 310.47
- MDL Number:
- MFCD00072475
- MOL File:
- 40596-69-8.mol
| Melting point | <25℃ |
|---|---|
| Boiling point | bp0.06 135-136° |
| Density | 0.9261 g/cm3 (20℃) |
| vapor pressure | 3.15 x l0-3 Pa (25 °C) |
| refractive index | 1.4200 (estimate) |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), Methanol (Sparingly) |
| form | Liquid |
| Water Solubility | 1.4 mg l-1 (room temperature) |
| color | Colorless to light yellow |
| BRN | 1913191 |
| InChIKey | NFGXHKASABOEEW-LDRANXPESA-N |
| CAS DataBase Reference | 40596-69-8 |
| FDA UNII | 8B830OJ2UX |
| EPA Substance Registry System | Methoprene (40596-69-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335-H411 |
| Precautionary statements | P261-P264-P271-P273-P302+P352-P305+P351+P338 |
| Hazard Codes | Xi,N |
| Risk Statements | 36/37/38-51/53 |
| Safety Statements | 26-36-60-61 |
| RIDADR | UN3082 9/PG 3 |
| WGK Germany | 2 |
| RTECS | JR1685000 |
| Hazardous Substances Data | 40596-69-8(Hazardous Substances Data) |
| Toxicity | LD50 orally in rats: >34500 mg/kg (Siddall) |
METHOPRENE price More Price(7)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 33375 | Methoprene PESTANAL?, analytical standard | 40596-69-8 | 100mg | $192 | 2024-03-01 | Buy |
| TRC | M260715 | Methoprene | 40596-69-8 | 250mg | $305 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | CHM0014480 | METHOPRENE 95.00% | 40596-69-8 | 5MG | $498.83 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | 41301 | Methoprene | 40596-69-8 | 25mg | $650 | 2021-12-16 | Buy |
| Crysdot | CD31001966 | Methoprene 98+% | 40596-69-8 | 50mg | $55 | 2021-12-16 | Buy |
METHOPRENE Chemical Properties,Uses,Production
Insect juvenile hormone
Methoprene is a kind of insect Juvenile hormone type biochemical insecticide developed by Novartis Co., Ltd. The juvenile hormone is capable of regulating the growth and development of insects and hormones themselves. The major role of juvenile hormone is to suppress the un-aged larvae metamorphosis, to maintain the larvae characteristics of juvenile insects, so that the larvae stage is still maintained after molting.
The methoprene act as a kind of tobacco leaf protectors and interfere with the peeling process of insects. It can interfere with the growth and development process of tobacco beetle and tobacco moth so that the imago would lose reproductive capacity, further effectively controlling the growth of the population of the tobacco leaf pest.
Information regarding the toxicity, application, and precautions of the insect-preserving hormone insecticides are edited by Tongtong from Chemicalbook (2017-03-04).
Toxicity
Acute oral LD50> 34 600mg/kg: body weight.
The preparation has a stimulating effect on the eyes, belonging to a low-toxicity pesticide.
Application
Juvenile hormone can not kill insects directly insects, can only make insects died in the metamorphosis process, or through infertility or eggs do not hatch to reduce the number of offspring population, it is slow, can not quickly control the risk of fulminant pests, Agricultural applications. At present mainly used in the following areas:
- Control of health pests. Ethylenolate has a high activity of German cockroaches, which makes both female and male adults sterile. Continue to use this drug treatment, six months to a year later, it can be inferred because of infertility, large cockroaches are also effective. It is also effective to control the fleas, mosquitoes and flies.
- Control of Hemiptera pests. Enzyme is effective in the control of greenhouse aphids and whitefly. But in the field application stability is not good. The control of greenhouse whitefly and scale insects is effective.
- Control pests. Proposed juvenile hormones have high activity against Lepidoptera pests during storage of cereals, flour, tobacco and so on. The United States tries to use insecticides and dioxins to be effective for many pests.
- Control of ants. The insect insect bait can block the normal larvae of the larvae, so that the king of infertility, prevention and treatment of kitchen ants and other effective. It is also useful to report juvenile hormone treatment of termites.
- Pollution-free new pesticides for the control of tobacco storage pests and beetles and other beetles.
Instructions
Storage Tobacco Tobacco Beetle, with 41% soluble powder 40,000 times the liquid, directly sprayed on the tobacco. To ensure uniform spray, completely cover the tobacco leaves, you can use quantitative dilution or special multi-directional ultra-low spray equipment.
The different growth and development stages of insects are not as sensitive to juvenile hormones. Larvae or nymphs are most sensitive at the end of age, poor sensitivity to other age. The larvae of Aedes aegypti were 100 times higher than those of 3rd instar larvae. Pupal phase is more sensitive. In the process of insect growth and development, select the appropriate time, with exogenous juvenile hormone treatment, destruction of insects in the normal hormone balance, so that abnormal abnormal metamorphosis, adult infertility or eggs can not hatch, so as to achieve the purpose of control and eradication of pests The The insects treated by the insects, the larvae of the fly larvae can normalize the pupae, but can not be normal feathers, or death, may also feather wings, can not fly.
The IC50 fennel was 0.48 μg/L for the larvae of Culex pipiens mosquito, and the ID50 of the pupa was 2.2 μg per pupa.
Precautions
- The agent has a strong flammability, is strictly prohibited without dilution Direct use of the goods, to stay away from the fire and high fever surface, keep the seal.
- This product has a stimulating effect on the eyes, should be careful spray.
References
1. Wang Yubing, Lu Yin spectrum editor. Pollution-free pesticide practical manual. Zhengzhou: Henan Science and Technology Press .2004.
2. Editorial Board of Chinese Agricultural Encyclopedia Editorial Committee of Pesticide Editors, Editorial Department of China Agricultural Encyclopedia.
Description
Methoprene is the common name for a racemic mixture of two enantiomers (R and S in a ratio of 1:1). The activity of the compound as a juvenile hormone (JH) mimic is restricted to the S enantiomer. Methoprene was the first insect growth regulator approved in the 1970s by the US Environmental Protection Agency after extensive studies showing low toxicity to vertebrates and rapid natural chemical degradation in the environment and through organism metabolism. Nowadays, it is one of the most widely used and successful insect growth regulators. Different products containing methoprene (e.g., pesticides, veterinary drugs) are commercially available in different forms (emulsifiable concentrates, granules, pellets, briquettes, aerosols, or sustained-release formulations). Some of these are applied to water for mosquito control whereas others are sprayed in areas where foods are stored to prevent insect infestations. Methoprene may be used in combination with other active insecticides to optimize pest control.
Chemical Properties
Amber colored liquid. Faint fruity odor.
Uses
ectoparasiticide
Uses
Methoprene is a broad-spectrum synthetic JH mimic, which
acts as an insect growth regulator (insecticide). It prevents
larval insect stages from undergoing metamorphosis to viable
adults and thus acts as a larvicide. Methoprene is considered
a biochemical pesticide because rather than controlling target
pests through direct toxicity, methoprene interferes with the
insect life cycle, preventing the insect from reaching maturity
or reproducing.
It is useful for control of a variety of insect pests including
ants, mosquitoes, flies, fleas, beetles, lice, and moths, but is
only effective against larvae, not adults or pupae. In order to
control some of these insects, methoprene is used in the
production of a number of foods including meat, milk,
mushrooms, peanuts, rice, and cereals. Many different products
(e.g., pesticides, veterinary drugs) and formulations containing
methoprene are commercially available. Methoprene products
used for protecting pets such as cats and dogs include capsules
administered orally and flea collars used externally. Production
animals (e.g., cattle) typically receive methoprene in the diet as
a food additive. Other formulations of methoprene include
emulsifiable concentrates, pellets and tablets, granules, and
aerosols. Some of these are applied to water for mosquito
control whereas others are sprayed in areas where foods are
stored to prevent insect infestations. A potential therapeutic use
of methoprene was proposed in the context of African sleeping
sickness, since it was observed that this compound killed
Trypanosoma brucei in culture. However, methoprene acid,
resulting from the insecticide metabolism, exhibited no efficiency
as trypanocide. Consistently, methoprene administered
to infected mice showed to be unable to eliminate trypanosomes
from the blood.
Uses
Methoprene controls many insect pests (Diptera, Pharaoh’s ants, and also Coleoptera, Homoptera and Siphonaptera) in public health, stored commodities, food handling, processing and storage establishments, mushroom houses, on animals and on plants (including glasshouse plants).
Definition
An insecticidal preparation said to act in the manner of a juvenile hormone, which arrests development of insects in the larval stage.
Agricultural Uses
Insect growth hormone: Methoprene is an insect growth regulator (IGR) used against a variety of insects including horn flies, mosquitoes, beetles, tobacco moths, sciarid flies, fleas (eggs and larvae), fire ants, pharoah ants, midge flies and Indian meal moths. Controlling some of these insects, methoprene is used in the production of a number of foods including meat, milk, mushrooms, peanuts, rice and cereals. It also has several uses on domestic animals (pets) for controlling fleas and to control insects in wastewater, sludge beds and ponds. For oral use in dogs, 9 weeks of age and older and 4 pounds body weight or greater, for the prevention and control of flea populations [21 CFR 520.1390]. Not approved for use in EU countries . Registered for use in the U.S.
Trade name
ALTOSID®; APEX®; DIACON®; DIANEX®; ENT 70,460®; EXTINGUISH®; FLEATROL®; KABAT®; MANTA®; MOORMAN’S® IGR CATTLE CONCENTRATE; OVITROL®; PHARORID®; PRECOR®; ZR-515®
Potential Exposure
Methoprene is a natural insect growth regulator (IGR) that mimics juvenile hormone(s) and is used against a variety of insects including horn flies, mosquitoes, beetles, tobacco moths, sciarid flies, fleas (eggs and larvae), fire ants, pharoah ants, midge flies and Indian meal moths. Controlling some of these insects, methoprene is used in the production of a number of foods including meat, milk, mushrooms, peanuts, rice and cereals. It also has several uses on domestic animals (pets) for controlling fleas and to control insects in wastewater, sludge beds and ponds. For oral use in dogs, 9 weeks of age and older and 4 lb body weight or greater, for the prevention and control of flea populations
Environmental Fate
Methoprene may be degraded by demethylation, hydrolysis,
oxidative cleavage, and photodegradation, resulting in the
formation of a series of metabolites that include methoprene
acid and citronellic acid. The primary modes of degradation are
photodegradation and degradation by aquatic microorganisms.
It is metabolized rapidly in soil under both aerobic and
anaerobic conditions (half-life = 10–14 days). The major
microbial degradation product is carbon dioxide. Degradation
in both freshwater and saltwater is also quite rapid with a halflife
of 10–35 days at 20 ℃. Methoprene is not very soluble in
water (<2 ppm) and as a result is not highly mobile in soil.
Because of this and its rapid biodegradation, methoprene does
not persist for long periods in soil and is unlikely to contaminate
groundwater. When released into water, methoprene is
expected to adsorb to suspended solids and sediment. A high
potential for bioconcentration in aquatic organisms has been
suggested, with an estimated bioconcentration factor of 3400.
However, studies with bluegill sunfish, showed no significant
bioconcentration of methoprene in fish tissues as a result of
aquatic exposure.
Methoprene rapidly degrades in plants, with a half-life of
1–2 days in alfalfa when applied at a rate of 1 pound per acre.
In rice, the half-life is less than 1 day. In wheat, its half-life was
reported to be 3–7 weeks, depending on the level of moisture
in the plant.
Metabolic pathway
Methoprene is readily degraded biologically by hydrolysis of the ester group, O-demethylation and oxidative cleavage of the bond at the 4-position.
Shipping
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required
Degradation
Methoprene (1) is stable in water and in the presence of aqueous acids and
alkalis. It is sensitive to UV light. Isomerisation of the double bond is
facile. In sunlight (S)-methoprene decomposes to a number of products.
These include trans,trans-( S)-methoprenic acid, 2-cis,4-trans-( S)-methoprenic
acid, and 2-cis,4-trans-(S)-methoprene.
When [5-14C]methoprene was irradiated in direct sunlight in Pyrex
vessels in aqueous solutions (0.01 ppm and 0.50 ppm) the DT50 was less
than one day. Initially decomposition was rapid, but after one week 12%
and 5% 1 remained in the 0.5 and 0.01 ppm solutions, respectively.
Carbon dioxide was collected and total 14C recovered was not less than
94% during a 21-day experiment. Five products were characterised as
oxygenated products but could not be positively identified. For product
identification, an aqueous emulsion of methoprene was irradiated in
sunlight and four photoproducts (24% yield) were characterised as methoxycitronellal
dimethyl acetal(3,3.9%), methoxycitronellic acid (4,4.7%),
an epoxide of methoprene (5,4%) and a methyl ketone (6,4%). In addition
to unreacted methoprene there were at least 46 other photoproducts
of which none represented more than 2% yield. Rose Bengal and
anthraquinone increased the rate of photocatalysed breakdown of
methoprene and the profile of products was similar to that obtained by
irradiation of a thin film. The extent of decomposition in the presence
of anthraquinone was 86% after 6 hours and the predominant product
was methoxycitronellal (2, l0-14%). Photosensitised oxidation was slow
and 47% of the original was unreacted and a single major product
(12%) was identified as the dihydrofuranol (7) (Quistad et al., 1975a)
(see Scheme 1).
Toxicity evaluation
Acute oral LD50 for rats: >34,600 mg/kg
Waste Disposal
It is the responsibility of chemical waste generators to determine if a discarded chemical is classified as a hazardous waste. See 40 CFR Parts 261.3 for United States Environmental Protection Agency guidelines for the classification determination. In addition, in order to ensure complete and accurate classification, waste generators must consult state and local hazardous waste regulations. Incineration might be an effective disposal procedure where permitted. If an efficient incinerator is not available, the product should be mixed with large amounts of combustible material and contact with the smoke should be avoided. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers
METHOPRENE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12837 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 | bess@weibangbio.com | China | 18152 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2986 | 55 |
| Nanjing Dolon Biotechnology Co.,Ltd. | 18905173768 | sales@dolonchem.com | CHINA | 2972 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
View Lastest Price from METHOPRENE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | Methoprene
40596-69-8
|
US $55.00-79.00 / mg | 95.32% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-10-30 | METHOPRENE
40596-69-8
|
US $0.00-0.00 / KG | 1KG | 99% | 500000kg | Hebei Weibang Biotechnology Co., Ltd | |
 |
2024-10-25 | METHOPRENE
40596-69-8
|
US $30.00-9.00 / kg | 1kg | 0.99 | 30 tons | Hebei Yanxi Chemical Co., Ltd. |
-

- Methoprene
40596-69-8
- US $55.00-79.00 / mg
- 95.32%
- TargetMol Chemicals Inc.
-

- METHOPRENE
40596-69-8
- US $0.00-0.00 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd
-

- METHOPRENE
40596-69-8
- US $30.00-9.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.