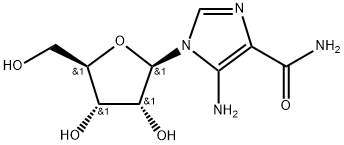
AICAR
- CAS No.
- 2627-69-2
- Chemical Name:
- AICAR
- Synonyms
- AAKG;AMPK;ACADESINE;AMPK1;PRKAB1;Acadesine, AICA;5-Aminoimidazole-4-carboxamide riboside;5-Aminoimidazole-4-carboxamide ribonucleoside;5-AMINOIMIDAZOLE-4-CARBOXAMIDE-1-B-D-RIBOFURANOSIDE;{[(2S,3R,4R,5R)-5-(5-aMino-4-carbaMoyl-1H-iMidazol-1-yl)-3,4-dihydroxyoxolan-2-yl]Methoxy}phosphonic acid
- CBNumber:
- CB1675294
- Molecular Formula:
- C9H14N4O5
- Molecular Weight:
- 258.23
- MDL Number:
- MFCD00869751
- MOL File:
- 2627-69-2.mol
- MSDS File:
- SDS
| Melting point | 214-215 °C |
|---|---|
| Boiling point | 726.3±60.0 °C(Predicted) |
| Density | 2.06±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | H2O: >10 mg/mL |
| form | powder |
| pka | 13.27±0.70(Predicted) |
| color | tan |
| Water Solubility | Soluble in DMSO at 2mg/ml. Soluble in water or ethanol at less than 1mg/ml |
| λmax | 265nm(NaOH)(lit.) |
| Merck | 14,16 |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20°C for up to 3 months. |
| InChIKey | RTRQQBHATOEIAF-UUOKFMHZSA-N |
| CAS DataBase Reference | 2627-69-2(CAS DataBase Reference) |
| FDA UNII | F0X88YW0YK |
| NCI Drug Dictionary | acadesine |
| ATC code | C01EB13 |
| EPA Substance Registry System | 1H-Imidazole-4-carboxamide, 5-amino-1-.beta.-D-ribofuranosyl- (2627-69-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H315-H319-H360D |
| Precautionary statements | P202-P264-P280-P302+P352-P305+P351+P338-P308+P313 |
| Hazard Codes | Xi,T |
| Risk Statements | 36/37/38-61 |
| Safety Statements | 26-36-45-53 |
| WGK Germany | 3 |
| TSCA | Yes |
| HS Code | 29349990 |
AICAR price More Price(80)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SRP5003 | AMPK (α2/β2/γ1), active, His tagged human PRECISIO? Kinase, recombinant, expressed in baculovirus infected Sf9 cells, ≥70% (SDS-PAGE), buffered aqueous glycerol solution | 2627-69-2 | 5μG | $887 | 2024-03-01 | Buy |
| Sigma-Aldrich | SAB1306034 | ANTI-AMPK ALPHA2(C-TERMINAL) antibody produced in rabbit IgG fraction of antiserum, buffered aqueous solution | 2627-69-2 | 400μL | $481 | 2024-03-01 | Buy |
| Sigma-Aldrich | A9978 | AICAR ≥98% (HPLC), powder | 2627-69-2 | 5mg | $80.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | SAB1306032 | ANTI-AMPK ALPHA 1(C-TERMINAL) antibody produced in rabbit IgG fraction of antiserum, buffered aqueous solution | 2627-69-2 | 400μl | $481 | 2024-03-01 | Buy |
| Sigma-Aldrich | A9978 | AICAR ≥98% (HPLC), powder | 2627-69-2 | 25mg | $279 | 2024-03-01 | Buy |
AICAR Chemical Properties,Uses,Production
adenosine regulating agents
Acadesine is the prototype of a new class of compounds termed adenosine regulating agents. Acadesine is a purine nucleosid analogue that enters the myocyte and is immediately phosphorylated to ZMP (AICA ribotide), which is further metabolised to Inosine mono-phosphate (an intermediate in the synthesis ofadenosine triphosphate (ATP) and guanosine triphos-phate).
Claims that acadesine may serve as a substrate for ATP synthesis and result in repletion of myocardial ATP were supported by some studies and refuted by others. Because acadesine may be a precursor in the synthesis of myocardial ATP it was proposed as a possible agent of myocardial protection during ischaemia, particularly because myocardial ATP depletion has been linked to cell death.
Description
AICAR (2627-69-2) activates AMP-activated protein kinase (AMPK). Promotes ligand-independent activation of the insulin receptor.1 Promotes skeletal muscle autophagy via activation of FoxO3a.2 Controls smooth muscle cell hyperproliferation in vascular disease.3 ?Induces osteogenic differentiation in mesenchymal stem cells.4 Inhibits proinflammatory response in glial cells.5 Cell permeable.
Chemical Properties
Solid
Originator
AICA,BIOMOL
Uses
glucose uptake stimulant; AMPK activator
Uses
AICAR is a nucleoside analogue that is able to enter nucleoside pools and is able to significantly increase levels of adenosine during periods of ATP breakdown. Adenosine-regulating agents (ARAs) hav e been recognized for therapeutic potential in myocardial ischemia. Cardioprotective.
Uses
5-Aminoimidazole-4-carboxamide 1-beta-D-ribofuranoside is used as a cell permeable activator of AMP-activated protein kinase (AMPK), a metabolic master regulator that is activated in times of reduced energy availability (high cellular AMP:ATP ratios) and serves to inhibit anabolic processes. In vivo, pharmacologic activation of AMPK with AICAR mimics exercise and triggers insulin-independent glucose uptake by skeletal muscle.
Definition
ChEBI: A 1-ribosylimidazolecarboxamide in which the carboxamide group is situated at position 4 of the imidazole ring, which is further substituted at position 5 by an amino group. A purine nucleoside analogue and activator of AMP-activated protein kinase, it is is used for the treatment of acute lymphoblastic leukemia and is reported to have cardioprotective effects.
Manufacturing Process
Adenosine 3', 5'-cyclic phosphate N'-oxide (76.0 g, 0.200 mole) as the dihydrate was dissolved in a solution of 400 ml DMSO and 31.0 g (0.204
mole) 1,5-diazabicyclo[5.4.0]undec-5-ene. The solution was cooled to 15°C
and 40 ml methyl iodide was added with stirring at room temperature. After
30 min, the mixture had gelled; 1.5 L ethanol was added and the solid was
thoroughly homogenized by vigorous stirring. The solid was filtered, and the
resulting paste was resuspended in 2 L ethanol and homogenized. The product
was again filtered, washed with ethanol and ether, and dried, giving 80.4 g of
1-methoxyadenosine 3',5'-cyclic phosphate suitable for further transformation
(recrystallization from aqueous methanol with ether).
A solution of 30.0 g 1-methoxyadenosine 3',5'-cyclic phosphate (81.5 mmole),
20.0 g NaHCO3 (238 mmole), and 300 ml H2O was refluxed 45 mm. The pH
of the solution was adjusted to 2.5 with Dowex 50x8 (H)+ while warm, and a
water pump vacuum was applied to mixture to remove CO2. The pH was
readjusted to 9-10 with NaOH, and the resin was removed by filtration. The
solution was passed onto a column containing 400 ml Dowex 1x2 (formate,
100-200 mesh), and the column was washed well with water. The column was
eluted with a gradient of 4 L water in the mixing chamber and 4 L 4 N formic
acid in the reservoir. The first major product, coming after about 2 L eluate,
was 5-amino-N-methoxy-1-β-D-ribofuranosylimidazole-4-carboxamidine 3',5'-
cyclic phosphate, giving 5.4 g (19%) after evaporation of the solvent and
trituration of the residue with ethanol (recrystallization from water).
A solution of 5.0 g (14.3 mmoles) 5-amino-N-methoxy-1-β-Dribofuranosylimidazole-
4-carboxamidine 3',5'-cyclic phosphate in 200 ml H2
preheated to 60°C and containing approximately 5.0 g moist sponge nickel
catalyst, was shaken with 2-3 atm. H2 at 60°C for 2 h. The filtered solution
was evaporated to dryness to give 3.75 g of 5-amino-1-β-Dribofuranosylimidazole-
4-carboxamidine 3',5'-cyclic phosphate (82%),
(recrystallization from water).
A mixture of 4.0 g (12.5 mmole) 5-amino-1-β-D-ribofuranosylimidazole-4-
carboxamidine 3',5'-cyclic phosphate and 100 ml conc. NH4OH was heated in
a bomb at 100°C for 16 h, then cooled and evaporated in vacuum. The
residue was taken up in 100 ml H2O and applied to a 2.5x20 cm column of
Dowex 1x2 (formate form, 100-200 mesh). After washing well with H2O the
column was eluted with a gradient of 1 L H2O in the mixing chamber and 1 L
3 N formic acid in the reservoir. Fractions containing the product, appearing
near the end of the elution, were evaporated. Trituration of the residue with
EtOH gave 2.90 g (68%) of 5-amino-1-β-D-ribofuranosylimidazole-4-
carboxamide 3',5'-cyclic phosphate.
The 5-amino-1-β-D-ribofuranosylimidazole-4-carboxamide may be produced
by hydrolysis of 5-amino-1-β-D-ribofuranosylimidazole-4-carboxamide 3',5'-
cyclic phosphate with NaOH.
Therapeutic Function
Cardiotonic, Platelet aggregation inhibitor
Biological Functions
The first direct AMPK activator, 5-aminoimidazole-4-carboxamide riboside (AICAR), is an adenosine analog taken up into cells by adenosine transporters and phosphorylated by adenosine kinase, thus generating the AMP-mimetic, AICAR monophosphate (ZMP). Similarly to cellular AMP, ZMP binds to site 3 on the AMPKγ subunit. Although ZMP is a much less potent AMPK activator than AMP in cell-free systems, AICAR directly activates AMPK in most cells because ZMP can accumulate to millimolar concentrations in cells. AICAR is able to activate many other AMP-dependent enzymes, such as fructose-1,6-bisphosphatase.
General Description
Adenosine monophosphate?protein kinase (AMPK) activator 5-aminoimidazole-4-carboxamide?ribonucleotide?(AICAR)?modulates cellular energy. It regulates lipid and glucose metabolism, pro-inflammatory responses,?cytokine production,?cell proliferation and apoptosis. AICAR improves ischemia or reperfusion injury and kidney fibrosis in rats. It provides protection against acute tubular necrosis.
Biological Activity
Cell-permeable, allosteric activator of AMP-activated protein kinase (AMPK). Augments proliferation, differentiation and mineralization of osteoblastic MC3T3-EI cells and attenuates psychosine-induced expression of proinflammatory cytokines and iNOS in astrocytes.
Biochem/physiol Actions
AICAR is a cell permeable activator of AMP-activated protein kinase (AMPK), a metabolic master regulator that is activated in times of reduced energy availability (high cellular AMP:ATP ratios) and serves to inhibit anabolic processes. In vivo, pharmacologic activation of AMPK with AICAR mimics exercise and triggers insulin-independent glucose uptake by skeletal muscle.
Side effects
AICAR is an AMPK (adenosine 5′-monophosphate (AMP)-activated protein kinase) activator that has been shown to have significant endurance-enhancing effects and is banned for use as a doping agent by the World Anti-Doping Agency. Overactivation of AMPK, or activation in the wrong tissues, can lead to serious side effects, including neurodegeneration or preventing cell division. Accumulation of naturally occurring AICAR in the body has also been linked to metabolic disorders in humans. However, AICAR has not been approved for human treatment and cannot be used as a drug. Therefore, its safety has yet to be verified.
storage
-20°C
Clinical claims and research
AICAR has proved in vivo anti-tumor effects in xenograft models. It is currently under clinical trial phase I/II to treat patients with chronic lymphocytic leukemia and has shown good safety and tolerability properties, although some side effects have been reported.
References
1) Chopra et al. (2012), Phosphorylation of the insulin receptor by AMP-activated protein kinase (AMPK) promotes ligand-independent activation of the insulin signalling pathway in rodent muscle; Diabetologia, 55 783 2) Sanchez et al. (2012), AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1; J. Cell Biochem., 113 695 3) Ferri et al. (2012), AMP-activated protein kinase and the control of smooth muscle cell hyperproliferation in vascular disease; Vascul. Pharmacol., 56 9 4) Wu et al. (2011) AICAR, a small chemical molecule, primes osteogenic differentiation of adult mesenchymal stem cells; Int. J. Artif. Organs, 34 1128 5) Giri et al. (2004) 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase; J. Neurosci., 24 479
AICAR Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Changzhou Xuanming Pharmaceutical Technology Co., Ltd. | +86-519-85525359 +86-15995072465 | sales@xuanmingchem.com | China | 914 | 58 |
| Zhuzhou New Peptides Tech Co.,Ltd. | +86-18073326374 +86-18073326374 | sales@goodpeptides.com | China | 56 | 58 |
| Hong Kong Excellence Biotechnology Co., Ltd. | ada@sh-teruiop.com | China | 875 | 58 | |
| Wuhan Ruichi Technology Co., Ltd | +8613545065237 | admin@whrchem.com | China | 153 | 58 |
| Shanghai Affida new material science and technology center | +undefined15081010295 | admin@oudaxin.com | China | 114 | 58 |
| Huaian Banting Trading Co., Ltd. | +86-13102576090 | sales5@bantingpeptide.com | China | 35 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Wuhan Han Sheng New Material Technology Co.,Ltd | +8617798174412 | admin01@hsnm.com.cn | China | 2097 | 58 |
Related articles
- Mechanism and Dosage of AICAR
- 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) is an intermediate in the generation of inosine monophosphate. AICAR is ....
- Apr 12,2022
- What is AICAR?
- AICAR (5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside) is a substance produced naturally by the body that stimulates AMP ....
- Mar 17,2022
Related Qustion
- Q:What is the therapeutic potential of AICAR
- A:The therapeutic potential of AICAR is due to the fact that the pathological processes that cause disturbances in the action of....
- Jul 3,2024
View Lastest Price from AICAR manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | AICAR
2627-69-2
|
US $60.00 / mg | 50mg | 99% | 20kg | Hebei Zhuanglai Chemical Trading Co.,Ltd | |
 |
2024-11-22 | AICAR
2627-69-2
|
US $60.00-50.00 / box | 1box | 99.99% | 500 box | hebei hongtan Biotechnology Co., Ltd | |
 |
2024-11-22 | AICAR
2627-69-2
|
US $60.00-45.00 / box | 1box | 99.99% | 1000 | hebei hongtan Biotechnology Co., Ltd |