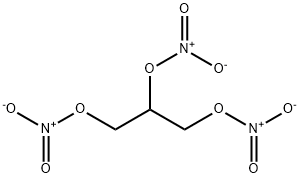
Nitroglycerin
- CAS No.
- 55-63-0
- Chemical Name:
- Nitroglycerin
- Synonyms
- Glyceryl trinitrate;NITROGLYCERINE;NTG;GTN;TNG;Nitrolingual;Diluted Nitroglycerin;Nitrol;Tridil;Anginine
- CBNumber:
- CB2145318
- Molecular Formula:
- C3H5N3O9
- Molecular Weight:
- 227.09
- MDL Number:
- MFCD00171649
- MOL File:
- 55-63-0.mol
| Melting point | 2.8°; mp 13.5° |
|---|---|
| Boiling point | 368.78°C (rough estimate) |
| Density | d1515 1.599; d44 1.6144; d415 1.6009; d425 1.5918 |
| refractive index | nD15 1.474 |
| Flash point | 12°C |
| storage temp. | -20°C |
| solubility | Miscible with acetone and with anhydrous ethanol |
| form | solution |
| Water Solubility | 1.25g/L(25 ºC) |
| Exposure limits | TLV-TWA skin 0.05 ppm (0.5 mg/m3) (ACGIH), 0.2 ppm (MSHA, OSHA, and NIOSH). |
| Dielectric constant | 19.0(20℃) |
| BCS Class | 1 |
| FDA 21 CFR | 250.102 |
| CAS DataBase Reference | 55-63-0(CAS DataBase Reference) |
| EWG's Food Scores | 2-6 |
| FDA UNII | G59M7S0WS3 |
| NCI Dictionary of Cancer Terms | nitroglycerin |
| NCI Drug Dictionary | nitroglycerin |
| ATC code | C01DA02,C01DA52,C05AE01 |
| NIST Chemistry Reference | 1,2,3-Propanetriol, trinitrate(55-63-0) |
| EPA Substance Registry System | Nitroglycerin (55-63-0) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H311 | |||||||||
| Precautionary statements | P264-P270-P280-P301+P312-P302+P352+P312-P361+P364 | |||||||||
| Hazard Codes | E,T+,N,Xn,T | |||||||||
| Risk Statements | 11-51/53-33-26/27/28-3-36-20/21/22-23/24/25 | |||||||||
| Safety Statements | 7-16-61-45-36/37-35-33-26 | |||||||||
| RIDADR | 1993 | |||||||||
| OEL | STEL: 0.1 mg/m3 [skin] | |||||||||
| WGK Germany | 3 | |||||||||
| HazardClass | 1.1A | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 2920900002 | |||||||||
| Hazardous Substances Data | 55-63-0(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 oral in rabbit: 1607mg/kg | |||||||||
| IDLA | 75 mg/m3 | |||||||||
| NFPA 704 |
|
Nitroglycerin Chemical Properties,Uses,Production
Chemical Properties
Nitroglycerin is a pale yellow oily liquid and also available in the form of rhombic crystals. It is highly explosive. It is used in combination with ethylene glycol dinitrite in the manufacture of dynamites. It is slightly soluble in water and miscible with acetone, ether, benzene, and other organic solvents. Nitroglycerin is incompatible with strong acids, such as hydrochloric acid, sulphuric acid, and nitric acid, and also with ozone and causes violent reactions. Nitroglycerin is a powerful explosive in its pure form and very sensitive to mechanical shock, heat, or UV radiation.
Uses
Glyceryl trinitrate (GTN; nitroglycerin) is a representative compound from the organic nitrate ester family. Introduced in 1879, it has been used as a therapeutic agent in the treatment of angina pectoris. In the later part of the nineteenth century, the explosive property of nitroglycerin was discovered, and it then became the active component of dynamite. Glyceryl trinitrate, ethylene glycol dinitrate, and other nitrate esters are commonly used in military and mining explosives. In preparing dynamite, these nitrate esters are absorbed in a dope of oxidizing salts and various inert fillers.
Description
Nitroglycerin is an oily, poisonous, clear to pale yellow, explosive liquid.Nitroglycerin is made by nitrating glycerol. Early industrial processes used a batch process in which glycerol was added to a mixture with approximately equal volumes of nitric acid and sulfuric acid.The sulfuric acid serves to ionize the nitric acid and removes water formed in the nitration process.
Chemical Properties
Nitroglycerin is a pale yellow liquid or crystalline solid (below 13℃).
Chemical Properties
Clear, colourless or slightly yellow solution
History
It was first prepared
in 1846 by the Italian chemist Ascanio Sobrero (1812–1888), who nitrated glycerol using a
mixture of nitric acid and sulfuric acid. Sobrero, who was injured in an explosion doing his
research, realized the compound’s danger and abandoned work on nitroglycerin. Twenty years
after Sobrero’s discovery, Alfred Nobel (1833–1896) developed its use commercially. Nobel
mixed nitroglycerin with other substances, searching for a safe way to transport it and make it less sensitive to heat and pressure.
nitroglycerin when it was first marketed,and Nobel continued to experiment with methods to
make nitroglycerin safer.One of these was mixing nitroglycerin with materials to make a solid
form of nitroglycerin. Nobel discovered that when nitroglycerin was mixed with a silica-based
diatomaceous earth material called kieselguhr,a relatively stable product resulted.The mixture
produced a paste that Nobel could pack into cardboard tubes;these could then be inserted
into holes drilled into rock structures and detonated.In 1867,Nobel patented his mixture and
called it dynamite,a name derived from the Greek word dunamis, meaning power.Nobel also
perfected a blasting cap made from mercury fulminate (Hg(ONC)2) and potassium chlorate
(KClO3) to detonate the nitroglycerin.
Uses
Vasodilator (coronary) Minitran (3M Pharmaceuticals); Nitro-Bid (Sanofi Aventis); Nitro-Dur (Key); Nitrol (Rorer); Nitrolingual (Pohl Boskamp); Nitrostat (Pfizer); Transderm-Nitro (Novartis).
Uses
Nitroglycerin is an explosive agent contained in dynamite and an antianginal and vasodilator treatment. It is a well-known irritant in dynamite manufacturers. It can also cause allergic reactions in employees of manufacturers of explosives, or in the pharmaceutical industries. Nitroglycerin can cross react with isosorbide dinitrate.
Uses
Nitroglycerin has medicinal use as a vasodilator. The main medical use of nitroglycerin is to treat angina pectoris. Nitroglycerin was first used to treat this condition in the late 19th century. It is prescribed today in various forms (tablet, ointment, patches, and injection) for patients who suffer from angina pectoris. Nitroglycerin is marketed under various trade names: Nitro-Dur, Nitrostat, Nitrospan, Nitro-Bid, and Tridil. When used in medications, the name glyceryl trinitrate is often used instead of nitroglycerin.
Definition
ChEBI: A nitroglycerol that is glycerol in which the hydrogen atoms of all three hydroxy groups are replaced by nitro groups. It acts as a prodrug, releasing nitric oxide to open blood vessels and so alleviate heart pain.
Production Methods
Nitroglycerin is made by nitrating glycerol.Early industrial processes used a batch processin which glycerol was added to a mixture with approximately equal volumes of nitric acidand sulfuric acid.The sulfuric acid serves to ionize the nitric acid and removes water formedin the nitration process.Removing the water formed in nitration increases the yield of nitroglycerin.Acids and water must be removed from the desired nitroglycerin through a washingprocess. The production of nitroglycerin is highly exothermic,and it is important to keepthe temperature below room temperature to prevent an explosion. Early production methodsused cooling coils in the nitration vessels to regulate the temperature.During the latter halfof the 20th century, safer continuous production methods replaced batch processes.In thesemethods much smaller reactors are required,as glycerol is reacted with the acids.
Definition
A highly explosive substance used in dynamite. It is obtained by treating glycerol (1,2,3-trihydroxypropane) with a mixture of concentrated nitric and sulfuric acids. It is not a nitro compound, but a nitrate ester CH2(NO3)CH(NO3)CH2(NO3).
Indications
Nitroglycerin (also isosorbide nitrate) relaxes isolated strips of human corpus cavernosum. Its mechanism involves the stimulation of guanylate cyclase. Clinically, nitroglycerin has been of limited use in the treatment of ED.
Synthesis Reference(s)
Tetrahedron, 49, p. 7037, 1993 DOI: 10.1016/S0040-4020(01)87978-3
General Description
Glyceryl trinitrate is the trinitrate ester ofglycerol and is listed as available in tablet form in the USP.It is prepared by carefully adding glycerin to a mixture of nitricand fuming sulfuric acids. This reaction is exothermic,and the reaction mixture must be cooled to between 10°Cand 20°C.The ester is a colorless oil, with a sweet, burning taste. Itis only slightly soluble in water, but it is soluble in organicsolvents.
.
General Description
Colorless to pale-yellow, viscous liquid or solid (below 56°F). (Note: An explosive ingredient in dynamite (20-40%) with ethylene glycol dinitrate (80-60%).).
Air & Water Reactions
Highly flammable.
Reactivity Profile
Nitroalkanes, such as NITROGLYCERIN, range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. Nitroalkanes are milder oxidizing agents, but still react violently with reducing agents at higher temperature and pressures. Nitroalkanes react with inorganic bases to form explosive salts. The presence of metal oxides increases the thermal sensitivity of nitroalkanes. Nitroalkanes with more than one nitro group are generally explosive. Nitroalkanes are insoluble in water. Flammable/combustible material. May be ignited by heat, sparks or flames. Nitroglycerin is incompatible with the following: Heat, ozone, shock, acids. Note: An OSHA Class A Explosive (1910.109). .
Hazard
Severe explosion risk, highly sensitive to shock and heat. Toxic by ingestion, inhalation, and skin absorption. Toxic by skin absorption. Vasodilator.
Health Hazard
Severe acute poisoning may result from ingestion of nitroglycerine or inhalation of its dust. The acute toxic symptoms include headache, nausea, vomiting, abdominal pain, tremor, dyspnea, paralysis, and convulsions. In addition, methemoglobinemia and cyanosis may occur. Ingestion of a relatively smallamount, 1.5-2.0 g, could be fatal to humans. Inhalation of its vapors or dust at 0.3 mg/m3 concentration in air produced an immediate fall in blood pressure and headache in human volunteers (ACGIH 1986). Chronic poisoning may produce headache and hallucination.
LD50 value, oral (rats): 105 mg/kg.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Contact allergens
Nitroglycerin is an explosive agent contained in dynamite and an antianginal and vasodilator treatment available in systemic and topical forms. It is a well known irritant agent in dynamite manufacture. It can also cause allergic reactions in employees of explosives manufacturers and in the pharmaceutical industry. Transdermal systems are the main source of iatrogenic sensitization. Nitroglycerin can cross-react with isosorbide dinitrate.
Mechanism of action
Nitroglycerine reduces the load on the heart by dilating peripheral veins, reducing the myocardial need for oxygen, and facilitating redistribution of coronary blood flow in the region of the myocardium with reduced blood flow.
Clinical Use
Nitroglycerin is used extensively as an explosive in dynamite.A solution of the ester, if spilled or allowed to evaporate,will leave a residue of nitroglycerin. To prevent anexplosion of the residue, the ester must be decomposed byadding alkali. Even so, the material dispensed is so dilutethat the risk of explosions does not exist. It has a strong vasodilatingaction and, because it is absorbed through theskin, is prone to cause headaches among workers associatedwith its manufacture. This transdermal penetration is whynitroglycerin is useful in a patch formulation. In medicine, ithas the action typical of nitrites, but its action develops moreslowly and is of longer duration. Of all the known coronaryvasodilatory drugs, nitroglycerin is the only one capable ofstimulating the production of coronary collateral circulationand the only one able to prevent experimental myocardialinfarction by coronary occlusion.
Side effects
Vascular headache, postural hypotension, and reflex
tachycardia are common side effects of organic nitrate
therapy. Fortunately, tolerance to nitrate-induced headache
develops after a few days of therapy. Postural hypotension
and tachycardia can be minimized by proper
dosage adjustment and by instructing the patient to sit down when taking rapidly acting preparations. An effective
dose of nitrate usually produces a fall in upright
systolic blood pressure of 10 mm Hg and a reflex rise in
heart rate of 10 beats per minute. Larger changes than
these should be avoided, because a reduction in myocardial
perfusion and an increase in cardiac oxygen requirements
may actually exacerbate the angina.
Since nitrite ions oxidize the iron atoms of hemoglobin
and convert it to methemoglobin, there may be a
loss in oxygen delivery to tissues. While methemoglobinemia
does not follow therapeutic doses of organic nitrates,
it can be observed after overdosage or accidental
poisoning.
Safety Profile
Human poison by an unspecified route. Poison experimentally by ingestion, intraperitoneal, subcutaneous, and intravenous routes. An experimental teratogen. Other experimental reproductive effects. A skin irritant. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. It can cause respiratory difficulties and death due to respiratory paralysis by ingestion. The acute symptoms of nitroglycerin poisoning are headaches, nausea, vomiting, abdominal cramps, convulsions, methemoglobinemia, circulatory collapse and reduced blood pressure, excitement, vertigo, fainting, respiratory rales, and cyanosis. Toxic effects may occur by ingestion, inhalation of dust, or absorption through intact skin. Human systemic effects by intravenous route: encephalitis, miosis, corneal damage. Used as a vasodilator and as an explosive. A very dangerous fire hazard when exposed to heat, flame, or by spontaneous chemical reaction. A severe explosion hazard when shocked or exposed to 03 , heat, or flame. Nitroglycerin is a powerful explosive, very sensitive to mechanical shock, heat, or UV radiation. Small quantities of it can readily be detonated by a hammer blow on a hard surface, particularly when it has been absorbed in filter paper. It explodes when heated to 215°C. Frozen nitroglycerin is somewhat less sensitive than the liquid. However, a half-thawed or partially thawed mixture is more sensitive than either one. When heated to decomposition it emits toxic fumes of NOx.
Synthesis
Nitroglycerine, 1,2,3-propantrioltrinitrate (19.1.1), is synthesized by nitrating glycerol with nitric acid.
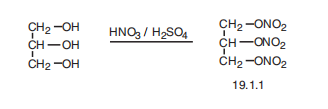
Potential Exposure
An explosive ingredient in dynamite (20-40%) with ethylene glycol dinitrate (80-60%). It is also used in making other explosives, rocket propellants; and medicine (vasodilator).
Drug interactions
Potentially hazardous interactions with other drugs
Anticoagulants: infusion of GTN reduces
anticoagulant effect of heparins.
Antidepressants: tricyclics may reduce effect of
sublingual tablets due to dry mouth.
Antimuscarinics: may reduce effect of sublingual
tablets due to dry mouth.
Avanafil, sildenafil, tadalafil, vardenafil: hypotensive
effect significantly enhanced - avoid concomitant
use.
Riociguat: avoid concomitant use due to risk of
hypotension.
Metabolism
Nitroglycerin is a lipid-soluble substance that is rapidly absorbed across the sublingual or buccal mucosa. Its onset of action occurs within 2 to 5 minutes, with maximal effects observed at 3 to 10 minutes. Little residual activity remains 20 to 30 minutes after sublingual administration. The plasma half-life of nitroglycerin, given sublingually or by spray, is estimated to be 1 to 3 minutes. Nitroglycerin and other organic nitrate esters undergo first-pass metabolism and are rapidly metabolized in the liver by the enzyme glutathione organic nitrate reductase.
Shipping
UN1204 Nitroglycerin solution in alcohol with not .1% nitroglycerin, Hazard Class: 3; Labels: 3-Flammable liquid. UN3064 Nitroglycerin, solution in alcohol with .1% but not .5% nitroglycerin, Hazard Class: 3; Labels: 3-Flammable liquid. UN0143 Nitroglycerin, desensitized with not ,40% nonvolatile, water-insoluble phlegmatizer, by mass. It falls in Hazard Class 1.1D (subsidiary hazard: 6.1).
Incompatibilities
Heat, ozone, shock, acids. An OSHA Class A Explosive (1910.109). Heating may cause violent combustion or explosion. May explosively decompose on shock, friction, or concussion. Reacts with ozone causing explosion hazard.
Waste Disposal
Do not wash into sewer. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.