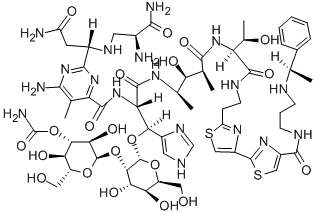
PEPLOMYCIN
- CAS No.
- 68247-85-8
- Chemical Name:
- PEPLOMYCIN
- Synonyms
- Pepleomycin;PLM;Fxyd1;Peplocin;PEPLOMYCIN;Pepleocine;NK-631{PEP;Pepreomycin;Bleomycin PEP;PEP-Bleomycin
- CBNumber:
- CB2180678
- Molecular Formula:
- C61H88N18O21S2
- Molecular Weight:
- 1473.59
- MDL Number:
- MFCD00866443
- MOL File:
- 68247-85-8.mol
| Density | 1.0908 (rough estimate) |
|---|---|
| refractive index | 1.6700 (estimate) |
| storage temp. | -20°C |
| FDA UNII | 56H9L80NIZ |
| NCI Drug Dictionary | peplomycin |
PEPLOMYCIN price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SAB1300001 | Monoclonal Anti-Phospho-PLM-S68 antibody produced in mouse purified immunoglobulin, buffered aqueous solution | 68247-85-8 | 100μG | $441 | 2024-03-01 | Buy |
| Usbiological | 481432 | FXYD1 | 68247-85-8 | 100ul | $499 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0012215 | PEPLOMYCIN 95.00% | 68247-85-8 | 5MG | $500.89 | 2021-12-16 | Buy |
| Biorbyt Ltd | orb596273 | FXYD1 | 68247-85-8 | 10μg | $290.7 | 2021-12-16 | Buy |
PEPLOMYCIN Chemical Properties,Uses,Production
Originator
Pepleo, Nippon Kayaku, Co., Japan ,1981
Uses
Antineoplastic.
Definition
ChEBI: Peplomycin is a glycopeptide.
Manufacturing Process
In 400 ml of dimethylformamide was dissolved 15.0 g of bleomycinic acid (copper-containing form). To the solution kept at 0°C by cooling were added 1.1 ml of N-methylmorpholine and 10.3 g of 6-chloro-1-pchlorobenzenesulfonyloxybenzotriazole (CCBT) as an activating compound. The mixture was stirred for 5 minutes at 0°C, then admixed with 5.3 g of N[(S)-1'-phenylethyl]-1,3-diaminopropane and further stirred for 1 hour.
After termination of the reaction by adding 200 ml of a 25% aqueous acetic acid solution, the reaction mixture was mixed with 5 liters of cold acetone to precipitate the reaction product. The precipitate was collected by filtration, washed with acetone, and dissolved in 500 ml of distilled water. The resulting aqueous solution was immediately adjusted to pH 6.0 and poured into a column containing 2 liters of CM-Sephadex C-25 (NH4+type) packed in 0.05 M aqueous ammonium chloride solution to adsorb bleomycins.
Using aqueous ammonium chloride solution, elution was performed by passing through the column 20 liters of eluent in which the concentration of ammonium chloride was continually increased from 0.05 to 1.0 M. The unreacted bleomycinic acid was found in the effluent at the ammonium chloride concentration of about 0.05 M and NK631 at the ammonium chloride concentration of about 0.45 M. Both fractions, which showed UV absorption at 292 nm;, were separately collected.
The NK631-containing fraction was poured into a resin column containing 2.6 liters of Amberlite XAD-2. The column was then washed thoroughly with water and eluted with 0.01 N hydrochloric acid in methanol-water (4:1 v/v). A total of 2.5 liters of the blue fraction, which showed UV absorption at 292 mμ, was collected. After evaporating off the methanol from the eluent fraction, the concentrate was adjusted to pH 6.0 with Dowex 44 (OH- type, an anionexchange resin composed of a copolymer of epichlorohydrin and ammonia) and was freeze-dried to obtain 16.1 g (92% yield) of NK631 dihydrochloride (copper-containing form) in the form of blue amorphous powder.
By similar treatment, 280 mg of the unreacted bleomycinic acid (coppercontaining form) were recovered.
In 200 ml of distilled water was dissolved 10.0 g of the NK631 dihydrochloride (copper-containing form). The solution was poured into a column containing 600 ml of Amberlite XAD-2 packed in distilled water. The column was washed successively with 2 liters of an aqueous solution containing 5% of EDTA-Na2, 2.5 liters of a 5% aqueous sodium sulfate solution, and 630 ml of distilled water.
The column was then eluted with 0.0025 N sulfuric acid in methanol-water mixture (1:1 v/v). A total of 900 ml of fractions containing a substance which showed UV absorption at 290 mμ was collected. After removal of methanol by distillation, the residual liquid was adjusted to pH 6.0 with Dowex 44 (OHtype) and freeze-dried to obtain 9.3 g (95% yield) of NK631 monosulfate (copper-free form) in the form of pale yellowish-white amorphous powder.
Therapeutic Function
Antineoplastic
PEPLOMYCIN Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
PEPLOMYCIN Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 22883 | 58 |
| Baoji Didu Pharmaceutical and Chemical Co., Ltd | 02961856358 15829046862 | 1035@dideu.com | China | 10011 | 58 |
| Supplier | Advantage |
|---|---|
| Dideu Industries Group Limited | 58 |
| Baoji Didu Pharmaceutical and Chemical Co., Ltd | 58 |
View Lastest Price from PEPLOMYCIN manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-07-02 | PEPLOMYCIN USP/EP/BP
68247-85-8
|
US $1.10 / g | 1g | 99.9% | 100 Tons Min | Dideu Industries Group Limited |
-

- PEPLOMYCIN USP/EP/BP
68247-85-8
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
68247-85-8(PEPLOMYCIN)Related Search:
1of4