β-Estradiol
- CAS No.
- 50-28-2
- Chemical Name:
- β-Estradiol
- Synonyms
- Estradiol;17β-estradiol;17β-estradiol;Oestradiol;17beta-Estradiol;Vagifem;Estrogel;17β-Oestradiol;Dihydrofolliculin;BETA-ESTRADIOL-16,16,17-D3
- CBNumber:
- CB2200244
- Molecular Formula:
- C18H24O2
- Molecular Weight:
- 272.39
- MDL Number:
- MFCD01074033
- MOL File:
- 50-28-2.mol
- MSDS File:
- SDS
| Melting point | 178-179 °C(lit.) |
|---|---|
| alpha | D25 +76 to +83° (dioxane) |
| Boiling point | 355.44°C (rough estimate) |
| Density | 1.0708 (rough estimate) |
| refractive index | 80.4 ° (C=1, Dioxane) |
| Flash point | 2℃ |
| storage temp. | room temp |
| solubility | Practically insoluble in water, soluble in acetone, sparingly soluble in ethanol (96 per cent), slightly soluble in methylene chloride. |
| pka | pKa 10.71±0.02(H2O(0.1% p-dioxane) t=25±0.1 I=0.03(KCl))(Approximate) |
| form | powder |
| color | White to off-white |
| Water Solubility | Soluble in dimethyl sulfoxide, ethanol , water, phosphate buffer saline, dimethyl formamide, acetone, dioxane and alkali hydroxides. Slightly soluble in vegetable oils. |
| Merck | 14,3703 |
| BRN | 1914275 |
| BCS Class | 1 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | VOXZDWNPVJITMN-ZBRFXRBCSA-N |
| CAS DataBase Reference | 50-28-2(CAS DataBase Reference) |
| EWG's Food Scores | 3-6 |
| NCI Dictionary of Cancer Terms | estradiol |
| FDA UNII | 4TI98Z838E |
| NCI Drug Dictionary | ESTRING |
| ATC code | G03CA03,G03CA53 |
| NIST Chemistry Reference | Estra-1,3,5(10)-triene-3,17beta-diol(50-28-2) |
| EPA Substance Registry System | Estradiol (50-28-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H351-H360FD-H362-H410 | |||||||||
| Precautionary statements | P202-P260-P263-P264-P273-P308+P313 | |||||||||
| Hazard Codes | T,Xn,F | |||||||||
| Risk Statements | 60-61-45-63-64-40-36-20/21/22-11-48 | |||||||||
| Safety Statements | 53-22-36/37/39-45-36/37-26-16-36-20 | |||||||||
| RIDADR | 2811 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | KG2975000 | |||||||||
| F | 8-10 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29372390 | |||||||||
| Toxicity | LD50 subcutaneous in rat: > 300mg/kg | |||||||||
| NFPA 704 |
|
β-Estradiol price More Price(81)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | E1132 | β-Estradiol analytical standard | 50-28-2 | 1vial | $131 | 2024-03-01 | Buy |
| Sigma-Aldrich | E1132 | β-Estradiol analytical standard | 50-28-2 | 5vials | $521 | 2024-03-01 | Buy |
| Sigma-Aldrich | E-060 | 17β-Estradiol solution 1.0?mg/mL in acetonitrile, ampule of 1?mL, certified reference material, Cerilliant? | 50-28-2 | 1mL | $142 | 2024-03-01 | Buy |
| Sigma-Aldrich | 3301 | 17β-Estradiol - CAS 50-28-2 - Calbiochem Most potent mammalian estrogenic hormone. | 50-28-2 | 1g | $82.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1250008 | Estradiol United States Pharmacopeia (USP) Reference Standard | 50-28-2 | 500mg | $436 | 2024-03-01 | Buy |
β-Estradiol Chemical Properties,Uses,Production
description
β-Estradiol is an endogenous estrogenic hormone receptor (ER) agonist (Ki values are 0.12 and 0.13 nM for ERα and ERβ respectively). Also high affinity ligand at membrane estrogen GPR30 receptors. β-Estradiol is an activator of PI 3-kinase.
Estradiol (17β-estradiol, β-Estradiol, E2, 17β-Oestradiol) is a human sex hormone and steroid, and the primary female sex hormone. Estradiol upregulates IL-6 expression through the estrogen receptor β (ERβ) pathway.
Uses
17β-Estradiol is the major estrogen secreted by the premenopausal ovary.This compound is a contaminant of emerging concern (CECs). Drinking water contaminant candidate list 3 (CCL 3) compound as per United States Environmental Protection Agency (EPA), environmental, and food contaminants.
β-Estradiol is used to study cell differentiation and transformations (tumorigenicity).
Indications and Usage
Estradiol is a white or milky white ordorless crystalline powder. It is soluble in dioxane and acetone, slightly soluble in ethanol, and insoluble in water.
Estradiol is the intermediate between estradiol valerate and estradiol benzoate, and it is a type of estrogen drug. It can be used to treat uterine functional bleeding, primary amenorrhea, menopausal syndrome, and prostate cancer. Estradiol can promote and adjust the normal growth of female sex organs and secondary sex characteristics, promote mammary duct maturation and growth, and aid in posseting. Estradiol can also be used in biochemical research.
Adverse reactions
In high dosages, estradiol can inhibit the release of anterior pituitary prolactin, thus decreasing breast milk secretion. However, nausea, vomiting and endometrial hyperplasia-induced bleeding may occur. Patients with liver or kidney failure should use with caution.
Contradictions
Do not use on breasts, vaginal area and vaginal mucosa.
Description
Estradiol, 17-beta- is an odorless white to yellow crystalline substance. Molecular weight = 272.42;Boiling point = (decomposes); Freezing/Meltingpoint = 173 - 179℃. Hazard Identification (based onNFPA-704 M Rating System): Health 2, Flammability 1,Reactivity 0. Insoluble in water.
Chemical Properties
Estradiol, 17-β-is an odorless white to yellow crystalline substance.
Chemical Properties
White or almost white, crystalline powder or colourless crystals.
Uses
17β-Estradiol is the major estrogen secreted by the premenopausal ovary.This compound is a contaminant of emerging concern (CECs). Drinking water contaminant candidate list 3 (CCL 3) compound as per United States Environmental Protection Agency (EPA), environmental, and food contaminants.
Uses
Estradiol USP (Estrace) is used to treat Breast cancer; prostatic carcinoma.
Application
β-Estradiol has been used:
for the in vitro maturation of bovine cumulus-oocyte complexes (COCs)
as a supplement in in vitro maturation medium (IVM), which is used as a control medium
in estrogen-induction assay
Definition
ChEBI: The 17beta-isomer of estradiol.
Acquired resistance
Estradiol is the most potent endogenous estrogen, exhibiting high affinity for the ER and high potency when administered parenterally. When administered orally, estradiol is promptly conjugated in the intestine and oxidatively metabolized by the liver, resulting in its low oral bioavailability and therapeutic effectiveness.
General Description
Estradiol, estra-1,3,5(10)-triene-3,17β-diol, is the most activeof the natural steroid estrogens. Although its 17β-OHgroup is vulnerable to bacterial and enzymatic oxidation toestrone, it can be temporarily protected as anester at C3 or C17, or permanently protected by adding a17α-alkyl group (e.g., 17α-ethinyl estradiol, the most commonlyused estrogen in oral contraceptives). The increasedoil solubility of the 17β-esters (relative to estradiol) permitsthe esters to remain in oil at the IM injection site for extendedperiods. These derivatives illustrate the principles of steroidmodification. Transdermal estradiolproducts avoid first-pass metabolism, allowing estradiol tobe as effective as oral estrogens for treating menopausalsymptoms. A new transdermal spray, Evamist, was approvedin 2007. Estradiol itself is typically not very effective orallybecause of rapid metabolism, but an oral formulation of micronizedestradiol that allows more rapid absorption of thedrug is available (Estrace). In addition to the oral and transdermalproducts, estradiol is also available in gel, cream, andvaginal ring formulations. The commercially available estradiolesters are the following:
Estradiol 3-acetate, USP (oral; vaginal ring)
Estradiol 17-valerate, USP (IM injection)
Estradiol 17-cypionate, USP (IM injection).
Hazard
A carcinogen (OSHA).
Biological Activity
Endogenous estrogen receptor (ER) agonist (K i values are 0.12 and 0.13 nM for ER α and ER β respectively). Also high affinity ligand at membrane estrogen GPR30 receptors.
Biochem/physiol Actions
The major estrogen secreted by the premenopausal ovary. Estrogens direct the development of the female phenotype in embryogenesis and during puberty by regulating gene transcription and, thus, protein synthesis. It also induces the production of gonadotropins which, in turn, induce ovulation. Exposure to estradiol increases breast cancer incidence and proliferation.
Contact allergens
Natural estradiol, used in transdermal systems for hormonal substitution, can induce allergic contact dermatitis, with the risk of systemic contact dermatitis after oral reintroduction.
Mechanism of action
The most potent naturally occurring estrogen in mammals. It is synthesized primarily in the ovary, and also in the testis, adrenal gland and placenta, and to a limited extent by peripheral tissues (e.g., liver, fat, and skeletal muscle) from androstenedione and testosterone. It is responsible for the development of secondary sex characteristics in the female at puberty (i.e., growth and development of the vagina, uterus and fallopian tubes, enlargement of the breasts, and growth and maturation of long bones).
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. A promoter. Human reproductive effects by ingestion: ferthty effects. Experimental reproductive effects. Human mutation data reported. A steroid hormone much used in medicine. When heated to decomposition it emits acrid smoke and irritating fumes.
Synthesis
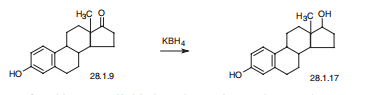
Estradiol, estra-1,3,5(10)-trien-3,17|?-diol (28.1.17), is most easily made by reducing the keto-group of estrone by various reducing agents, in particular potassium borohydride.
Potential Exposure
The working environment may be contaminated during sex hormone manufacture, especially during the extraction and purification of natural steroid hormones; grinding of raw materials; handling of powdered products and recrystallization. Airborne particles of sex hormones may be absorbed through the skin, ingested or inhaled. Enteric absorption results in quick inactivation of sex hormones in the liver. The rate of inactivation is decreased for the oral, alkylated steroid hormones (methyl testosterone, anabolic steroids, etc.). Sex hormones may accumulate and reach relatively high levels even if their absorption is intermittent. Consequently, repeated absorption of small amounts may be detrimental to health. Intoxication by sex hormones may occur in almost all the exposed workers if preventive measures are not taken. The effect in the industrial sector is more successful than the agricultural one (chemical caponizing of cockerels by stilbestrol implants and incorporation of estrogens in feed for body weight gain promotion in beef cattle), where measures taken are summary and the number of cases of intoxication is consequently bigger
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
storage
Room temperature
Shipping
UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
17-Estradiol (previously known as -estradiol) is purified by chromatography on SiO2 (toluene/EtOAc 4:1) and recrystallised from CHCl3/hexane or 80% EtOH. It is stable in air, is insoluble in H2O, and is precipitated by digitonin. The UV has max at 225 and 280 nm. The diacetate [3434-88-6] has m 97-98o and forms leaflets from aqueous EtOH. The 3-benzoate crystallises from aqueous MeOH withm 193o and [] D 25 +58o to 63o (c 1, dioxane). [Meischer & Scholz Helv Chim Acta 20 263, 1237 1937, Biochem J 32 1273 1938, Oppolzer & Roberts Helv Chim Acta 63 1703 1980, Inhoffen & Zühlsdorff Chem Ber 7 4 1914 1941, Beilstein 6 IV 6611.]
β-Estradiol Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hong Kong Excellence Biotechnology Co., Ltd. | ada@sh-teruiop.com | China | 875 | 58 | |
| Wuhan Nutra Biotechnology Co.,Ltd | +8617786394783 | nutrabiotech@outlook.com | China | 300 | 58 |
| Wuhan senwayer century chemical Co.,Ltd | +undefined-27-86652399 +undefined13627115097 | market02@senwayer.com | China | 902 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +86-15271838296; +8615271838296 | kyra@quanjinci.com | China | 1512 | 58 |
| Shandong Hanjiang Chemical Co., Ltd | +86-0533-2066820 +8618369939125 | hanson@sdhanjiang.com | China | 999 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 | info@fortunachem.com | China | 5986 | 58 |
| Shaanxi Xianhe Biotech Co., Ltd | +8617709210191 | Jerry@xhobio.com | China | 484 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | 1022@dideu.com | China | 3882 | 58 |
| Dorne Chemical Technology co. LTD | +86-86-13583358881 +8618560316533 | Ethan@dornechem.com | China | 3105 | 58 |
| Hebei Lingding Biotechnology Co., Ltd. | +86-18031140164 +86-19933155420 | erin@hbldbiotech.com | China | 17 | 58 |
Related articles
- β-Estradiol: Biosynthesis and Mechanism of Action
- β-Estradiol, synthesized from cholesterol via aromatase, acts through ERs (ERα, ERβ) and GPER, exerting genomic and non-genomi....
- Jun 26,2024
- Synthesis of β-estradiol
- β-estradiol is white or milky white leaf-like or needle-like crystals (ethanol solution), odorless. Beta-β-estradiol is stable....
- Mar 28,2022
- The effects of low estradiol
- Estradiol is used to treat menopause symptoms such as hot flashes and vaginal changes, and to prevent osteoporosis (bone loss)....
- Dec 15,2021
View Lastest Price from β-Estradiol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-03 | β-ESTRADIOL Estradiol
50-28-2
|
US $1.00 / g | 1g | 99% | 20 tons | Dorne Chemical Technology co. LTD | |
 |
2024-11-02 | Estradiol
50-28-2
|
US $0.00 / KG | 1KG | 97.0 ~ 103.0%; USP39 | 1000kg/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-02 | Estradiol
50-28-2
|
US $40.00 / kg | 1kg | 99.9% | 10000 | Hebei Miaoyin Technology Co.,Ltd |
-

- β-ESTRADIOL Estradiol
50-28-2
- US $1.00 / g
- 99%
- Dorne Chemical Technology co. LTD
-

- Estradiol
50-28-2
- US $0.00 / KG
- 97.0 ~ 103.0%; USP39
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Estradiol
50-28-2
- US $40.00 / kg
- 99.9%
- Hebei Miaoyin Technology Co.,Ltd
50-28-2(β-Estradiol)Related Search:
1of4