Pomalidomide
- CAS No.
- 19171-19-8
- Chemical Name:
- Pomalidomide
- Synonyms
- 4-AMino-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione;CC-4047;Pomadomide;Pomalidomide-d4;PomaL;IMiD 3;CS-388;ActiMid;Pomalomide;Pomalidomide
- CBNumber:
- CB32128749
- Molecular Formula:
- C13H11N3O4
- Molecular Weight:
- 273.24
- MDL Number:
- MFCD12756407
- MOL File:
- 19171-19-8.mol
- MSDS File:
- SDS
| Melting point | 318.5 - 320.5° |
|---|---|
| Boiling point | 582.9±45.0 °C(Predicted) |
| Density | 1.570±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥14mg/mL |
| pka | 10.75±0.40(Predicted) |
| form | powder |
| color | yellow |
| Merck | 14,135 |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. |
| InChI | InChI=1S/C13H11N3O4/c14-7-3-1-2-6-10(7)13(20)16(12(6)19)8-4-5-9(17)15-11(8)18/h1-3,8H,4-5,14H2,(H,15,17,18) |
| InChIKey | UVSMNLNDYGZFPF-UHFFFAOYSA-N |
| SMILES | C1(=O)C2=C(C(N)=CC=C2)C(=O)N1C1CCC(=O)NC1=O |
| NCI Dictionary of Cancer Terms | CC-4047; pomalidomide |
| FDA UNII | D2UX06XLB5 |
| NCI Drug Dictionary | pomalidomide |
| ATC code | L04AX06 |
| UNSPSC Code | 12352200 |
| NACRES | NA.77 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H360D | |||||||||
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | NR3397905 | |||||||||
| HS Code | 29251900 | |||||||||
| Hazardous Substances Data | 19171-19-8(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
Pomalidomide price More Price(61)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | P0018 | Pomalidomide ≥98% (HPLC) | 19171-19-8 | 5mg | $147 | 2024-03-01 | Buy |
| Sigma-Aldrich | P0018 | Pomalidomide ≥98% | 19171-19-8 | 25mg | $581 | 2024-03-01 | Buy |
| TCI Chemical | P2074 | Pomalidomide >98.0%(HPLC) | 19171-19-8 | 25mg | $18 | 2024-03-01 | Buy |
| TCI Chemical | P2074 | Pomalidomide >98.0%(HPLC) | 19171-19-8 | 100mg | $43 | 2024-03-01 | Buy |
| Cayman Chemical | 19877 | Pomalidomide ≥98% | 19171-19-8 | 1mg | $32 | 2024-03-01 | Buy |
Pomalidomide Chemical Properties,Uses,Production
Description
Pomalidomide inhibits LPS-induced TNF-α release with IC50 of 13 nM in PBMCs.
In vitro
Pomalidomide inhibits lipopolysaccharide (LPS) stimulated TNF-alpha release in human PBMC and in human whole blood with IC50 values of 13 nM and 25 nM, respectively. Pomalidomide inhibits the growth of T regulatory cells which is stimulated by IL-2 with an IC50 of ~1 μM. Treatment with Pomalidomide (6.4 nM-10 μM) increases the production of IL-2 in human peripheral blood T cells, and is slightly more potent in the CD4+ subset than in the CD8+ subset. Pomalidomide is significantly more potent than CC-5013 at elevating IL-2, IL-5, and IL-10 levels, but only slightly more potent than CC-5013 at elevating IFN-γ levels.
Pomalidomide enhances SEE and Raji cells induced AP-1 transcriptional activity in Jurkat cells in a dose-dependent manner, with a maximal enhancement of 4-fold at 1 μM. Exposure of Raji cells to various concentrations of Pomalidomide (2.5-40 μg/mL) for 48 hours leads to a significant decrease in cell proliferation and DNA synthesis. There is a reduction of ~40% compared to vehicle-treated controls.
In vivo
Pomalidomide enhances the antitumor effect of rituximab against B-cell lymphomas in severe combined immunodeficient mice. Administration of Pomalidomide in combination with rituximab, gives the mice a median survival period of 74 days compared with 58 days of CC5013/rituximab treatment and 45 days of rituximab nonotherapy. The synergistic effect of Pomalidomide and rituximab can be completely abrogated by depletion of NK cells, supporting the proposal that NK cell expansion is one mechanism by which Pomalidomide may augment rituximab antitumor activity.
Description
In February 2013, the US FDA approved pomalidomide (also known as CC4047) for the treatment of multiple myeloma (MM) in patients with disease progression after receiving other cancer therapeutics. Pomalidomide is a 4-amino analog of thalidomide with enhanced potency and an improved toxicity profile. Pomalidomide and thalidomide exert their effects by modulation of immunity, inhibition of angiogenesis, interference with the bone/tumor microenvironment, and inhibition of the cereblon protein. Pomalidomide potently inhibited in vitro proliferation in a variety of human MM cell lines, IC50~10 nM, while thalidomide showed almost no inhibition up to 100 μM. In mouse MM tumor models, 50 mg/kg daily doses of pomalidomide resulted in marked inhibition of tumor growth after 15 days of treatment and complete regression in 3–6 weeks versus thalidomide-treated controls at the same dose. Pomalidomide is prepared by condensation of 4-nitrophthalic anhydride with 3-aminopiperidine-2,6-dione followed by catalytic hydrogenation of the nitro group.
Chemical Properties
Yellow Solid
Originator
Celgene Corporation (United States)
Uses
Pomalidomide inhibits LPS-induced TNF-α release with IC50 of 13 nM
Uses
Pomalidomide is a second generation immunomodulator, TNF-α inhibitor, and thalidomide analog. An inhibitor of LPS-induced TNFαrelease.
Uses
Pomalidomide is a thalidomide derivative, a potent inhibitor of TNF-α production. It is an antiinflammatory and antitumor agent used in the treatment of multiple myeloma.
Definition
ChEBI: Pomalidomide is an aromatic amine that is thalidomide substituted at position 4 on the isoindole ring system by an amino group. Used for the treatment of multiple myeloma in patients who failed to respond to previous therapies. It has a role as an antineoplastic agent, an immunomodulator and an angiogenesis inhibitor. It is a dicarboximide, a member of isoindoles, a member of piperidones and an aromatic amine. It is functionally related to a thalidomide.
brand name
Pomalyst
reaction suitability
reagent type: ligand
Biochem/physiol Actions
Pomalidomide is an effective fetal hemoglobin (HbF) inducer that downregulates the key γ-globin repressors, SRY-box transcription factor 6 (SOX6), and BAF chromatin remodeling complex subunit (BCL11A).
Clinical Use
Treatment of multiple myeloma
Synthesis
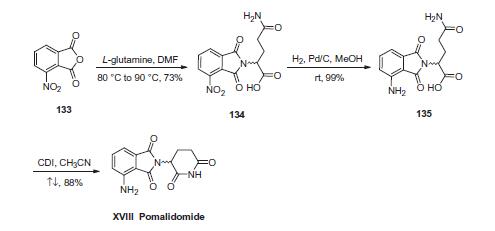
First, condensation of commercially available 3-nitrophthalic anhydride (133) and L-glutamine in warm DMF gave nitrophthalimide 134. Although the authors from Celgene do not explicitly describe the racemization of the stereocenter derived from L-glutamine, scrambling of the stereocenter has been reported during this step under neutral conditions at elevated temperatures. Next, hydrogenative reduction of the nitro group furnished the anilinophthalimide 135, and this was followed by treatment with CDI in refluxing acetonitrile to secure the piperidone dione and ultimately furnish pomalidomide (XVIII) as the racemate in 87% overall yield from 134.
target
TNF-α
Drug interactions
Potentially hazardous interactions with other drugs
Antidepressants: concentration increased by
fluvoxamine.
Metabolism
Mainly metabolised in the liver by the cytochrome P450
isoenzymes CYP1A2 and CYP3A4, with CYP2C19 and
CYP2D6 playing a minor role.
Following a single oral administration of
[14C]-pomalidomide (2 mg) to healthy subjects,
approximately 73% and 15% of the radioactive dose
was eliminated in urine and faeces, respectively, with
approximately 2% and 8% of the dosed radiocarbon
eliminated as pomalidomide in urine and faeces.
storage
Store at +4°C
References
1) A LOPEZ-GIRONA. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide[J]. Leukemia, 2012, 26 11: 2326-2335. DOI:10.1038/leu.2012.119.
2) Zhu?et al.?(2013),?Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma; Leukemia Lymphoma,?54?683
3) KATHERINE A DONOVAN. Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane Radial Ray syndrome.[J]. eLife, 2018. DOI:10.7554/eLife.38430.
4) Winter?et al.?(2015),?DRUG DEVELOPMENT. Phthalimide conjunction as a strategy for in vivo target protein degradation; Science,?348?1376
5) JASMIN LOHBECK Aubry K M. Practical synthesis of a phthalimide-based Cereblon ligand to enable PROTAC development[J]. Bioorganic & Medicinal Chemistry Letters, 2016, 26 21: Pages 5260-5262. DOI:10.1016/j.bmcl.2016.09.048.
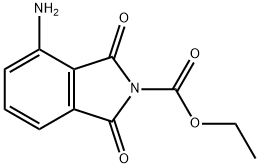
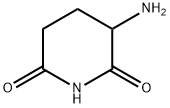
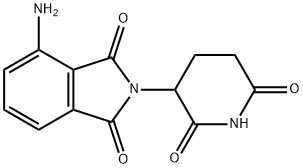
Pomalidomide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Beijing Mesochem Technology Co.,Ltd | +8613651027935 | rachel@mesochem.com | China | 191 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5868 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659 +86-13153181156 | sales@sdperfect.com | China | 294 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 997 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-17396673057 | linda@tnjone.com | China | 1143 | 58 |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 | sales@sjar-tech.com | China | 485 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29730 | 60 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1803 | 55 |
View Lastest Price from Pomalidomide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-24 | Pomalidomide
19171-19-8
|
US $0.00 / Kg/Bag | 10g | 99%min HPLC | 10KGS | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2025-04-23 | Pomalidomide
19171-19-8
|
US $10.00 / KG | 1KG | 99% | 10 mt | Hebei Chuanghai Biotechnology Co., Ltd | |
 |
2025-04-21 | Pomalidomide
19171-19-8
|
US $0.00-0.00 / kg | 1kg | 98 | 1000 | Wuhan Circle Star Chem-medical Technology Co.,Ltd |
-

- Pomalidomide
19171-19-8
- US $0.00 / Kg/Bag
- 99%min HPLC
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Pomalidomide
19171-19-8
- US $10.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
-

- Pomalidomide
19171-19-8
- US $0.00-0.00 / kg
- 98
- Wuhan Circle Star Chem-medical Technology Co.,Ltd





