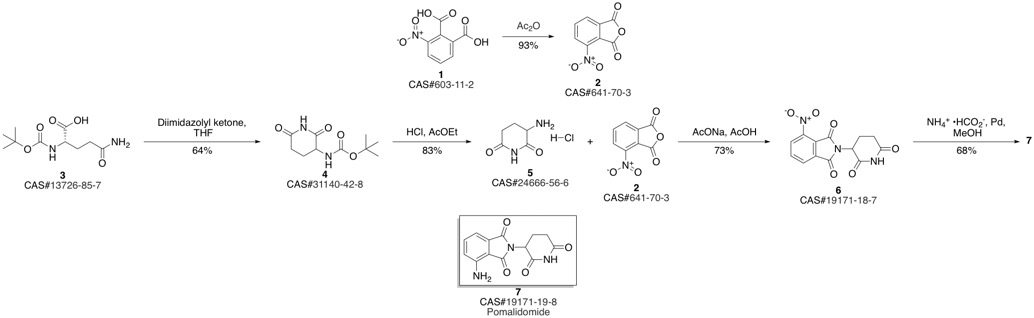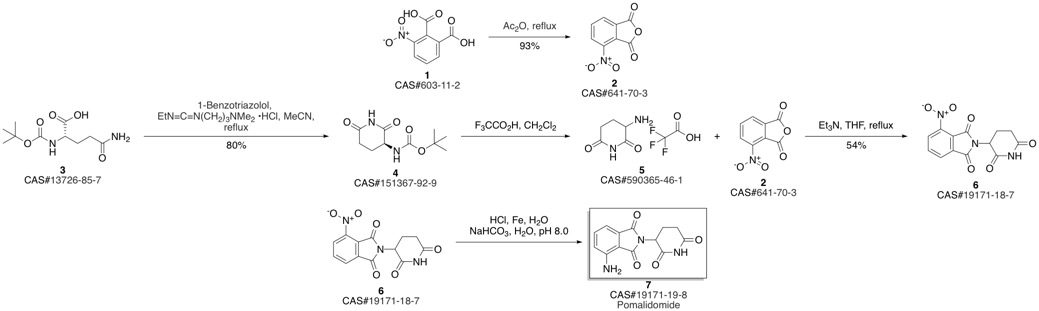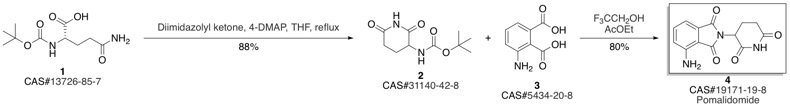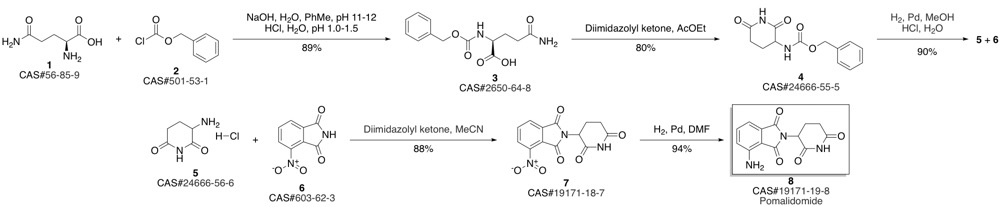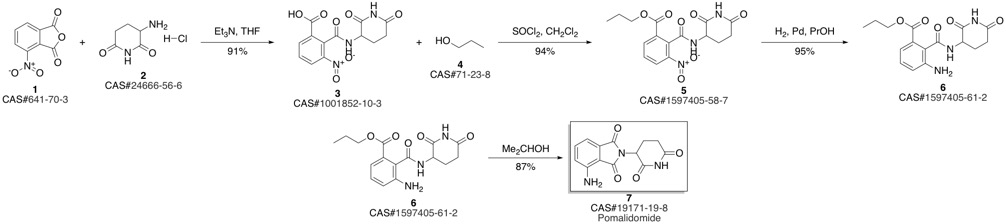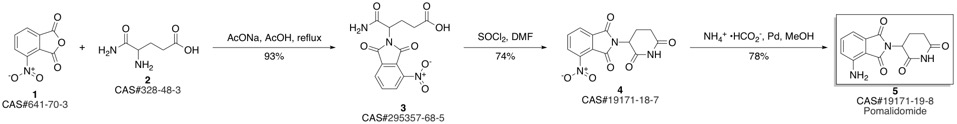
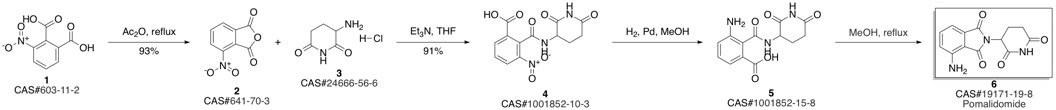
Pomalidomide synthesis
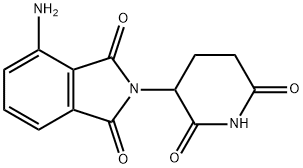
- Product Name:Pomalidomide
- CAS Number:19171-19-8
- Molecular formula:C13H11N3O4
- Molecular Weight:273.24
Huang, Daowei; Shen, Chengwu; Wang, Wenya; Huang, Lei; Ni, Feng; Li, Jianqi. New synthesis route for the preparation of pomalidomide. Synthetic Communications. Volume 46. Issue 16. Pages 1343-1348. Journal; Online Computer File. (2016).
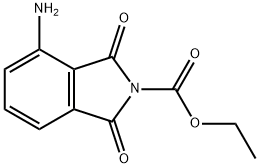
340020-02-2
0 suppliers
inquiry
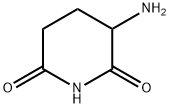
2353-44-8
104 suppliers
$27.00/5g

19171-19-8
498 suppliers
$5.00/100mg
Yield:19171-19-8 96.6%
Reaction Conditions:
with sodium acetate in acetonitrile; for 4 h;Reflux;
Steps:
2 Example 2 The production (R/S)-4-amino-2-(2,6-dioxopiperidin-3-yl)-1H-isoindole-1,3(2H)-dione (1)
40.0 g (170.8 mmol) of ethyl 4-amino-1,3-dioxo-1,3-dihydro-2H-isomdole-2-carboxylate, 800 cm3 of acetonitrile, 28.4 g (342.0 mmol) of anhydrous sodium acetate, 28.1 g (170.7 mmol) of 3-aminopiperidine-2,6-dione are measured into a 1000 cm3 round-bottomed flask, then the suspension obtained in this way is heated to reflux temperature. The stirring is continued at unchanged temperature for 4 hours. The reaction mixture is concentrated to about 40 cm3 at 40 °C with the help of a vacuum. 800 cm3 of distilled water is added to the concentrated residue, which is then stirred at room temperature for 30 minutes. (The water may also be added at an earlier stage.) Following this the crystalline product is filtered, washed with 2x400 cm3 of distilled water, then dried at 50 °C in a vacuum until constant weight is achieved. In this way 45.10 g (96.6%) of the product according to the title is obtained. Mp. : 314-315 °C (decomposes) IR (KBr): 3481 , 3378, 3248, 1752, 1703, 1635, 1362, 1197 cm 1. 1H NMR (DMSO-d6, 400 MHz): δ = 11.10 (b, 1H), 7.47 (m, 1H), 7.02 (m, 1H), 7.00 (m, 1H), 5.06 (m, 1H), 2.89 (m, 1H), 2.58 (m, 1H), 2.56 (m, 1H), 2.03 (m, 1H) ppm. HPLC: Waters Acquity UPLC BEH C18; 2.1 x50 mm; 1.7 μm; 0.5 mL/min; 225 nm; 10/90 CH3CN/0.1 % HClO4 (aq); 1.38 min (99.90%).
References:
WO2017/134476,2017,A1 Location in patent:Page/Page column 16

19171-18-7
117 suppliers
inquiry

19171-19-8
498 suppliers
$5.00/100mg
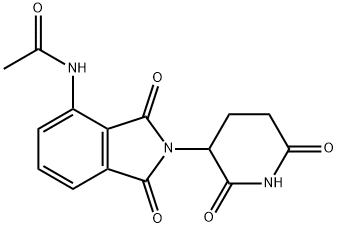
444289-17-2
1 suppliers
inquiry

19171-19-8
498 suppliers
$5.00/100mg
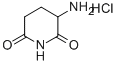
24666-56-6
454 suppliers
$8.00/5g
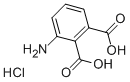
6946-22-1
183 suppliers
$33.10/1g

19171-19-8
498 suppliers
$5.00/100mg

24666-56-6
454 suppliers
$8.00/5g

19171-19-8
498 suppliers
$5.00/100mg