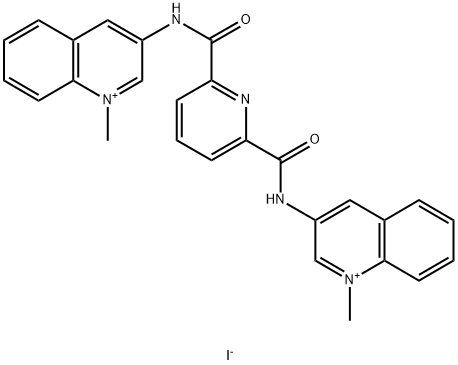
360 A iodide
- CAS No.
- 737763-37-0
- Chemical Name:
- 360 A iodide
- Synonyms
- 360 A iodide;360A (iodide);3,3'-[2,6-Pyridinediylbis(carbonylimino)]bis[1-methylquinolinium] diiodide
- CBNumber:
- CB32666580
- Molecular Formula:
- C27H23IN5O2+
- Molecular Weight:
- 576.42
- MDL Number:
- MOL File:
- 737763-37-0.mol
360 A iodide price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| ApexBio Technology | A3120 | 360Aiodide | 737763-37-0 | 5mg | $230 | 2021-12-16 | Buy |
| ChemScene | CS-1560 | 360Aiodide ≥98.0% | 737763-37-0 | 5mg | $240 | 2021-12-16 | Buy |
| ApexBio Technology | A3120 | 360Aiodide | 737763-37-0 | 10mg | $350 | 2021-12-16 | Buy |
| ChemScene | CS-1560 | 360Aiodide ≥98.0% | 737763-37-0 | 10mg | $384 | 2021-12-16 | Buy |
| ApexBio Technology | A3120 | 360Aiodide | 737763-37-0 | 25mg | $650 | 2021-12-16 | Buy |
360 A iodide Chemical Properties,Uses,Production
Biological Activity
360a is a 2,6-pyridine-dicarboxamide derivative displaying strong affinity and selectivity for g-quadruplex structures and selective telomerase inhibition in vitro assays. 360a is a g-quadruplex ligand, which can influence the consequence of g-quadruplex formation and/or stabilization.
in vitro
we found a s-phase accumulation in atm-proficient, but not in atm-deficient ebv-lymphocytes treated with 360a before induction of cell death. however, atm status did not modify cell cycle distribution in 360a-treated sv40-fibroblasts and hela cells compared to dmso treated controls [1]. dna-pkcs-dependent nhej was responsible for sister telomere fusions as a direct consequence of g-quadruplex formation and/or stabilization induced by 360a on parental telomere g strands. nhej and hr activation at telomeres altered mitotic progression in treated cells [2]. this compound was shown to display a potent affinity and selectivity for telomeric g-quadruplex dna over duplex dna and to induce delayed growth inhibition in ht1080 tumor cell line [3].
360 A iodide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32760 | 60 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131980 | 58 |
| Chembest Research Laboratories Limited | 021-20908456 | sales@BioChemBest.com | China | 6008 | 61 |
| Haoyuan Chemexpress Co., Ltd. | 021-58950125 | info@chemexpress.com | China | 7553 | 61 |
| MedChemexpress LLC | 021-58955995 | sales@medchemexpress.cn | United States | 4863 | 58 |
| AdooQ BioScience, LLC | +1 (866) 930-6790 | info@adooq.com | United States | 2784 | 58 |
| Guangzhou QiYun Biotechnology Co., Ltd. | 020-61288194 61288195 | 505721671@qq.com | China | 3868 | 58 |
| Musechem | +1-800-259-7612 | info@musechem.com | United States | 4662 | 60 |