- 360A iodide
-

- $1390.00 / 100mg
-
2024-10-24
- CAS:737763-37-0
- Min. Order:
- Purity:
- Supply Ability: 10g
|
| | 360 A iodide Basic information |
| Product Name: | 360 A iodide | | Synonyms: | 360 A iodide;360A (iodide);3,3'-[2,6-Pyridinediylbis(carbonylimino)]bis[1-methylquinolinium] diiodide | | CAS: | 737763-37-0 | | MF: | C27H23IN5O2+ | | MW: | 576.42 | | EINECS: | | | Product Categories: | | | Mol File: | 737763-37-0.mol | 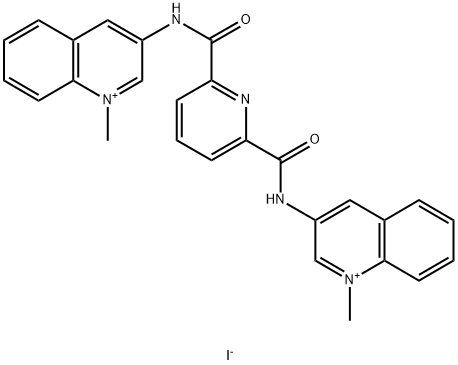 |
| | 360 A iodide Chemical Properties |
| storage temp. | Store at -20°C | | solubility | insoluble in H2O; insoluble in EtOH; insoluble in DMSO | | form | Solid | | color | Light yellow to yellow |
| | 360 A iodide Usage And Synthesis |
| Biological Activity | 360a is a 2,6-pyridine-dicarboxamide derivative displaying strong affinity and selectivity for g-quadruplex structures and selective telomerase inhibition in vitro assays. 360a is a g-quadruplex ligand, which can influence the consequence of g-quadruplex formation and/or stabilization. | | in vitro | we found a s-phase accumulation in atm-proficient, but not in atm-deficient ebv-lymphocytes treated with 360a before induction of cell death. however, atm status did not modify cell cycle distribution in 360a-treated sv40-fibroblasts and hela cells compared to dmso treated controls [1]. dna-pkcs-dependent nhej was responsible for sister telomere fusions as a direct consequence of g-quadruplex formation and/or stabilization induced by 360a on parental telomere g strands. nhej and hr activation at telomeres altered mitotic progression in treated cells [2]. this compound was shown to display a potent affinity and selectivity for telomeric g-quadruplex dna over duplex dna and to induce delayed growth inhibition in ht1080 tumor cell line [3]. |
| | 360 A iodide Preparation Products And Raw materials |
|