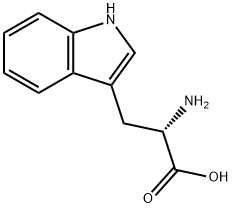
L-Tryptophan
- CAS No.
- 73-22-3
- Chemical Name:
- L-Tryptophan
- Synonyms
- TRYPTOPHAN;TRP;H-TRP-OH;TRYPTOPHANE;L-TRYPTOPHANE;L-Trp;L-Trp-OH;(s)-2-amino-3-(1h-indol-3-yl)propanoic acid;H-DL-TRP-OH;DL-TRYPTOPHANE
- CBNumber:
- CB3750054
- Molecular Formula:
- C11H12N2O2
- Molecular Weight:
- 204.23
- MDL Number:
- MFCD00064340
- MOL File:
- 73-22-3.mol
- MSDS File:
- SDS
| Melting point | 289-290 °C (dec.)(lit.) |
|---|---|
| alpha | -31.1 º (c=1, H20) |
| Boiling point | 342.72°C (rough estimate) |
| Density | 1.34 |
| refractive index | -32 ° (C=1, H2O) |
| storage temp. | 2-8°C |
| solubility | 20% NH3: 0.1 g/mL at 20 °C, clear, colorless |
| form | powder |
| pka | 2.46(at 25℃) |
| color | White to yellow-white |
| PH | 5.5-7.0 (10g/l, H2O, 20℃) |
| Odor | wh. cryst. or cryst. odorless powd., sl. bitter taste |
| optical activity | [α]20/D 31.5±1°, c = 1% in H2O |
| Water Solubility | 11.4 g/L (25 ºC) |
| Merck | 14,9797 |
| BRN | 86197 |
| Stability | Stable. Incompatible with strong acids, strong oxidizing agents. |
| InChIKey | QIVBCDIJIAJPQS-VIFPVBQESA-N |
| LogP | 0.704 (est) |
| FDA 21 CFR | 582.5915; 172.320; 310.545 |
| Substances Added to Food (formerly EAFUS) | L-TRYPTOPHAN |
| CAS DataBase Reference | 73-22-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 8DUH1N11BX |
| ATC code | N06AX02 |
| NIST Chemistry Reference | L-Tryptophan(73-22-3) |
| EPA Substance Registry System | L-Tryptophan (73-22-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS02 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314-H225-H290 | |||||||||
| Precautionary statements | P501-P240-P210-P233-P234-P243-P241-P242-P264-P280-P370+P378-P390-P303+P361+P353-P301+P330+P331-P363-P304+P340+P310-P305+P351+P338+P310-P403+P235-P406-P405 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 33-40-62-41-37/38-36/37/38-22 | |||||||||
| Safety Statements | 24/25-36/37/39-36-26 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | YN6130000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29339990 | |||||||||
| Toxicity | LD508mmol / kg (rat, intraperitoneal injection). It is safe when used in food (FDA, §172.320, 2000). | |||||||||
| NFPA 704 |
|
L-Tryptophan price More Price(83)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.16017 | (S)-(-)-Tryptophan for synthesis | 73-22-3 | 25g | $46.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.16017 | (S)-(-)-Tryptophan for synthesis | 73-22-3 | 100g | $161 | 2024-03-01 | Buy |
| Sigma-Aldrich | 6540-M | L-Tryptophan-CAS73-22-3-Calbiochem | 73-22-3 | 100g | $191 | 2024-03-01 | Buy |
| Sigma-Aldrich | 51145 | L-Tryptophan certified reference material, TraceCERT | 73-22-3 | 100mg | $134 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1700501 | L-Tryptophan United States Pharmacopeia (USP) Reference Standard | 73-22-3 | 200mg | $519 | 2024-03-01 | Buy |
L-Tryptophan Chemical Properties,Uses,Production
Description
As an essential amino acid, L-Tryptophan is necessary for normal growth in infants and for nitrogen balance in adults, which cannot be synthesized from more basic substances in humans and other animals, suggesting that it is obtained only by intake of tryptophan or tryptophan-containing proteins for human body, which is particularly plentiful in chocolate, oats, milk, cottage cheese, red meat, eggs, fish, poultry, sesame, almonds, buckwheat, spirulina, and peanuts, etc. It can be used as a nutritional supplement for use as an antidepressant, anxiolytic, and sleep aid. Thus, L-Tryptophan can be used for depression, anxiety, sleep apnea, premenstrual syndrome and many other problems. Besides, it can also be used in managing pain tolerance and managing weight.
It works by increasing the levels of certain neurotransmitters in the brain called serotonin. People suffer from depression have an imbalance of serotonin and other brain chemicals. Thus, the increase of serotonin levels in the brain can improve symptoms of depression. L-Tryptopan serves as the precursor for the synthesis of serotonin, which is converted to serotonin in the body. As a result, the symptoms of depression and other problems are improved.
Overview
It is also known as α-aminoindolylpropionic acid. Appearance: white to slightly yellowish white crystal or crystalline powder. No smell; Slight bitter taste. Melting temperature: 289 ° C (decomposition); soluble in water. Applications: as food fortifier, antioxidant; also used in medicine. It is manufactured from indole aldehyde, alternatively also be synthesized from trypsin decomposition and synthesis.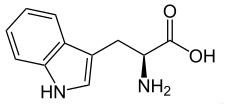
Content analysis
Accurately weigh about 300 mg sample and dissolve it in 3ml formic acid and 50ml glacial acetic acid; add 2 drops crystal violet test solution (TS-250), titrate with 0.1mol / L perchloric acid to the green end point or until the blue color completely disappears. Each Ml of 0.1 mol / L perchloric acid corresponds to 20.42 mg of L-tryptophan (C11H12N2O2).
Application
Amino acids-type drug:
It can be used in amino acid infusion, being often combined with iron and vitamins. Its co-administration with VB6 can improve depression and prevention/treatment of skin disease; as a sleep sedative, it can be combined with L-dopa for the treatment of Parkinson's disease. It is carcinogenic to experimental animals; it may cause adverse reactions including nausea, anorexia and asthmas. Avoid combination with monoamine oxidase inhibitors.
Nutritional supplements:
Tryptophan contained in egg white protein, fish meat, corn meal and other amino acids are limited; content in cereals such as rice is also low. It can be combined with lysine, methionine and threonine for enhanced amino acids. It can be supplemented to corn product at the content of 0.02% tryptophan and 0.1% lysine, being capable of significantly improving the protein potency.
References
https://en.wikipedia.org/wiki/Tryptophan
http://www.medicinenet.com/tryptophan-oral_capsule_tablet/article.htm
http://bodyandhealth.canada.com/drug/getdrug/apo-tryptophan
https://www.drugbank.ca/drugs/DB00150
Chemical Properties
White to off-white crystalline powder
Chemical Properties
Appearance: white crystalline powder. Odorless, slightly bitter taste;
Solubility: slightly soluble in water (1.14%, 25 ℃), insoluble in ethanol; soluble in dilute acid or dilute alkali. mp 289 ° C (decomposition).
Isoelectric point: 5.89. [α] D + 2.8 (5 mol / L HCl), [α] D + 6.2 (0.5 mol / L NaOH).
It can be colored upon long-term exposure to light. Its co-heating reaction with water will generate a small amount of indole while heating in the presence of sodium hydroxide and copper sulfate will produce a large amount of indole. It is stable upon heating together with acid in the dark, but is easily to be decomposed when heated together with other amino acids, carbohydrates and aldehydes. L-tryptophan will be completely decomposed when applying acid to break down the protein.
Uses
adrenergic agonist
Uses
tryptophan is one of the 21 amino acids comprising a protein. Tryptophan is a component of the skin’s natural moisturizing factors.
Uses
L-tryptophan is an essential amino acid which is necessary for normal growth in infants and for nitrogen balance in adults. It acts as a natural dietary supplement and used as an antidepressant, anxiolytic and sleep aid. It is used as a precursor to niacin, indole alkaloids and serotonin. It acts as an important intrinsic fluorescent probe, which finds to estimate the nature of microenvironment of the tryptophan.
Definition
ChEBI: L-tryptophan is the L-enantiomer of tryptophan. It has a role as an antidepressant, a nutraceutical, a micronutrient, a plant metabolite, a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is an erythrose 4-phosphate/phosphoenolpyruvate family amino acid, a proteinogenic amino acid, a tryptophan and a L-alpha-amino acid. It is a conjugate base of a L-tryptophanium. It is a conjugate acid of a L-tryptophanate. It is an enantiomer of a D-tryptophan. It is a tautomer of a L-tryptophan zwitterion.
Production Methods
The most attractive production processes for tryptophan are based on microorganisms used as enzyme sources or as overproducers: Enzymatic production from various precursors; Fermentative production from precursors; Direct fermentative production from carbohydrates by auxotrophic and analogue resistant regulatory mutants. L-tryptophan is synthesized from indole, pyruvate, and ammonia by the enzyme tryptophanase or from indole and L-serine/D,L-serine by tryptophan synthase these process variants were not economic due to the high costs of the starting materials. The microbial conversion of biosynthetic intermediates such as indole or anthranilic acid to L-tryptophan has also been considered as alternative for production.
However, the manufacturer using genetically modified strains derived fromBacillus amyloliquefaciens IAM 1521 was forced to stop Ltryptophan production. L-Tryptophan produced by this process was stigmatized because of side products found in the product causing a new severe disease termed eosinophilia-myalgia syndrome (EMS). One of the problematic impurities, "Peak E", was identified as 1,10-ethylidene- bis-(L-tryptophan), a product formed by condensation of one molecule acetaldehyde with two molecules of tryptophan.
brand name
Ardeytropin;Kalma;Optimax;Sedanoct.
World Health Organization (WHO)
L-tryptophan, an essential aminoacid and precursor of serotonin, was introduced into medicine in 1963 for the treatment of depression and sleep disorders. Its effectiveness in these conditions has, however, never been convincingly demonstrated. It is also widely used in dietary supplements, parenteral nutrition preparations and dietary products for children with phenylketonuria. In 1989, reports from the USA showed an association between the consumption of L-tryptophan containing preparations and the development of eosiniphilia-myalgia syndrome (EMS), a condition characterized by intense eosinophilia, severe muscle and joint pain, swelling of the arms and legs, skin rashes and possible fever. Some of the reported cases have been fatal. Since it is not yet clear whether L-tryptophan itself or an unidentified contaminant is the cause of the EMS, many drug regulatory authorities have suspended the marketing authorization of products containing tryptophan pending further investigation, whereas others have withdrawn these products or restricted their use.
Synthesis Reference(s)
The Journal of Organic Chemistry, 49, p. 3711, 1984 DOI: 10.1021/jo00194a008
General Description
White powder with a flat taste. An essential amino acid; occurs in isomeric forms.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition L-Tryptophan emits toxic fumes.
Fire Hazard
Flash point data for L-Tryptophan are not available. L-Tryptophan is probably combustible.
Biochem/physiol Actions
Tryptophan (Trp) is one of the functional amino acids that are associated with growth, reproduction, maintenance and immunity. Increased Trp availability is necessary for the regulation of mood, cognition and behaviour. It is hypothesised that L-Trp might be useful in inducing sleep in healthy adults against the normal circadian rhythm. Trp uptake by the brain depends on the plasma ratio of Trp to all of the other LNAAs (large neutral amino acids). Higher the Trp:LNAAs ratio, greater is the Trp uptake.
Safety Profile
Moderately toxic by intraperitoneal route. Experimental teratogenic and reproductive effects. Human mutation data reported. Questionable carcinogen with experimental tumorigenic data. When heated to decomposition it emits toxic fumes of NOx.
Purification Methods
Crystallise L-tryptophan from H2O/EtOH, wash it with anhydrous diethyl ether and dry it at room temperature in a vacuum over P2O5. It sublimes at 220-230o/0.03mm with 99% recovery and unracemised [Gross & Gradsky J Am Chem Soc 77 1678 1955]. [Cox & King Org Synth Coll Vol II 612 1943, Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2316-2345 1961, Beilstein 22 IV 6765.]
L-Tryptophan Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17365 | 58 |
| DONBOO AMINO ACID COMPANY | +8613063595538 | donboo@donboo.com | China | 9363 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12841 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 | info@fortunachem.com | China | 5978 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 988 | 58 |
| Wuhan Han Sheng New Material Technology Co.,Ltd | +8617798174412 | admin01@hsnm.com.cn | China | 2097 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Across Biotech Jinan Co LTD | +8613031735486 | frank@acrossbiotech.com | China | 105 | 58 |
Related articles
- The Multifaceted Role of L-Tryptophan and Its Derived Natural Products
- L-Tryptophan's versatility drives biosynthesis of diverse neuroactive compounds in Psilocybe mushrooms, showcasing its pivotal....
- Oct 23,2024
- L-Tryptophan:Mechanism of action,Safety concerns
- L-tryptophan is an amino acid. Amino acids are protein building blocks. L-tryptophan is called an "essential" amino acid becau....
- Oct 23,2019
View Lastest Price from L-Tryptophan manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-29 | L-Tryptophan
73-22-3
|
US $0.00-0.00 / Kg/Drum | 1KG | 98.5%-101.5% | 10 TONS | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-29 | L-Tryptophan
73-22-3
|
US $0.00-0.00 / Kg/Bag | 1Kg/Bag | 99% up, High Density | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-11-29 | L-Tryptophan
73-22-3
|
US $999.00-666.00 / kg | 1kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD |
-

- L-Tryptophan
73-22-3
- US $0.00-0.00 / Kg/Drum
- 98.5%-101.5%
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- L-Tryptophan
73-22-3
- US $0.00-0.00 / Kg/Bag
- 99% up, High Density
- Sinoway Industrial co., ltd.
-

- L-Tryptophan
73-22-3
- US $999.00-666.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
73-22-3(L-Tryptophan)Related Search:
1of4