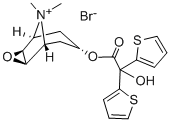
Tiotropium bromide
- CAS No.
- 136310-93-5
- Chemical Name:
- Tiotropium bromide
- Synonyms
- TiotropiuM broMide anhydrous;(1α,2β,4β,5α,7β)-7-[(Hy-droxydi-2-thienylacetyl)axy]-9,9-dimethyl-3-oxa-9-azoniatri-cyclo[3.3.1.02.4]nonane;(1a,2b,4b,5a,7b)-7-[(Hydroxydi-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]nonane bromide;(1a,2,4,5a,7)-7-[(2-Hydroxy-2,2-di-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]nonane Bromide;(1R,2R,4S,5S,7s)-7-(2-Hydroxy-2,2-di(thiophen-2-yl)acetoxy)-9,9-diMethyl-3-oxa-9-azatricyclo[3.3.1.02,4]nonan-9-iuM broMide;(1R,2R,4S,5S,7s)-7-[(2-Hydroxy-2,2-dithiophen-2-ylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]nonane bromide monohydrate;Tiova;Ba-679;BA 79BR;Spiriva
- CBNumber:
- CB4244930
- Molecular Formula:
- C19H22BrNO4S2
- Molecular Weight:
- 472.42
- MDL Number:
- MFCD00867027
- MOL File:
- 136310-93-5.mol
- MSDS File:
- SDS
| Melting point | 218-2200C |
|---|---|
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
| λmax | 240nm(lit.) |
| Merck | 14,9454 |
| CAS DataBase Reference | 136310-93-5(CAS DataBase Reference) |
| FDA UNII | XX112XZP0J |
| ATC code | R03BB04,R03BB54 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H360-H315-H319 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P | |||||||||
| HS Code | 2942.00.0500 | |||||||||
| NFPA 704 |
|
Tiotropium bromide price More Price(46)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TCI Chemical | T3032 | Tiotropium Bromide >98.0%(HPLC)(T) | 136310-93-5 | 50mg | $129 | 2024-03-01 | Buy |
| TCI Chemical | T3032 | Tiotropium Bromide >98.0%(HPLC)(T) | 136310-93-5 | 200mg | $323 | 2024-03-01 | Buy |
| Cayman Chemical | 15773 | Tiotropium (bromide) ≥98% | 136310-93-5 | 1mg | $73 | 2024-03-01 | Buy |
| Cayman Chemical | 15773 | Tiotropium (bromide) ≥98% | 136310-93-5 | 5mg | $141 | 2024-03-01 | Buy |
| Cayman Chemical | 15773 | Tiotropium (bromide) ≥98% | 136310-93-5 | 25mg | $431 | 2024-03-01 | Buy |
Tiotropium bromide Chemical Properties,Uses,Production
Description
Tiotropium bromide is a long-acting inhaled muscarinic antagonist, developed for the once-daily treatment of chronic obstructive pulmonary disease. Tiotropium bromide can be prepared in three steps. The Grignard condensation of 2-thienyl magnesium bromide with oxalic acid dimethyl ester, followed by a transesterlfication with scopine provided the ester which was quaternized with methyl bromide. Tiotropium bromide binds to human recombinant muscarinic receptors M1-, M2- and M3-subtypes with high and similar affinity, comparable to those obtained with ipratropium. Tiotropium bromide is characterized by its novel property of kinetic selectivity : while ipratropium rapidly dissociated from each of the receptor subtypes, tiotropium dissociated rapidly from M2 receptors (t1/2=3.6 h) but slowly from MI (t1/2=14.6 h) and M3 (t1/2=34.7 h) receptors. Inhibition of cholinergic bronchospasm by tiotropium bromide was demonstrated in anesthetized guinea pigs, rabbits and dogs. In healthy volunteers, inhalation of tiotropium bromide resulted in an absolute bioavailability of 19.5%, a t,,, value of 5 min. and the terminal half-life value of 5-6 days. There was no evidence of drug accumulation after repeated administration. The extent of biotransfonation was small with a urinary excretion of 74% of unchanged substance after iv. administration. Long term studies in patients with stable COPD have demonstrated that tiotropium bromide gave an effective bronchodilation that was maintained over 24h, significantly improved lung function as measured by FEVI (+ll-12%) and showed progressive reduction in dyspnea. It also reduced exacerbations of COPD patients and improved quality of life. Tiotropium bromide produced greater and more sustained bronchodilation than ipratropium bromide. Tiotropium has been shown to cause superior bronchodilatation and symptomatic improvements when compared to twice daily salmeterol in COPD. Tiotropium bromide was well tolerated and caused few adverse effects. The most common side effect reported was the mechanism-related effect of dry mouth.
Description
Tiotropium is an antagonist that binds to M1, M2, and M3 muscarinic acetylcholine receptors (Kds = 0.43, 0.54, and 0.69 nM, respectively, for human receptors). It decreases acetylcholine-induced contraction of isolated guinea pig trachea in a concentration-dependent manner. In vivo, tiotropium (1 g/L inhaled aerosol) confers complete protection against acetylcholine-induced bronchospasms in anesthetized dogs. Formulations containing tiotropium have been used in the treatment of chronic obstructive pulmonary disease (COPD).
Chemical Properties
White Solid
Originator
Boehringer lngelheim (Germany)
Uses
Anti - Asthmatic
Uses
Specially selective choline drug resistance
Uses
Muscarinic receptor antagonist. Bronchodilator
Definition
ChEBI: An organic bromide salt having (1alpha,2beta,4beta,5alpha,7beta)-7-[(hydroxydi-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]non ne as the counterion. Used (in the form of the hydrate) for maintenance treatment of airflow obstruction in patients with chronic obstructive pulmonary disease.
brand name
Spiriva
General Description
Tiotropium bromide, (1 ,2 ,4 ,7 )-7-[(hydroxidi-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]nonane, (Spiriva) is anantimuscarinic agent that is used in an inhalation device to deliverthe drug into the lungs. It is indicated in the treatment ofchronic obstructive pulmonary disease (COPD), includingchronic bronchitis and emphysema. The standard once-dailydose is 18 g of tiotropium.
Pharmacokinetics
Tiotropium is administered as a dry powder via inhalation using a HandiHaler, in which is placed the drug, contained in a green capsule. Patients should be cautioned not to be confused and take the medication orally. Systemic distribution following oral inhalation is minimal, essentially because of its hydrophilic character. If swallowed, only approximately 14% of the dose is eliminated in the urine, with the remainder being found in the feces. Inhaled tiotropium has a 30-minute onset of action but a much longer duration of action than ipratropium (24 versus <4 hours, respectively). Tiotropium is metabolized by both CYP3A4 and CYP2D6, followed by glutathione conjugation to a variety of metabolites. Only a very small amount is nonenzymatically hydrolyzed to inactive products.
Clinical Use
Tiotropium is the dithienyl derivative of N-methyl scopolamine, a quaternary analogue of naturally occurring scopolamine in Atropa belladonna. It is indicated primarily for the relief of bronchospasms associated with COPD and can be considered to be a site-specific, local medication to the lung.
Side effects
Tiotropium has an adverse reaction profile similar to that of ipratropium, with dry mouth being the most common adverse effect; however, blurred vision, tachycardia, urinary difficulty, headache precipitation, and exacerbation of narrow-angle glaucoma have been reported.
Synthesis
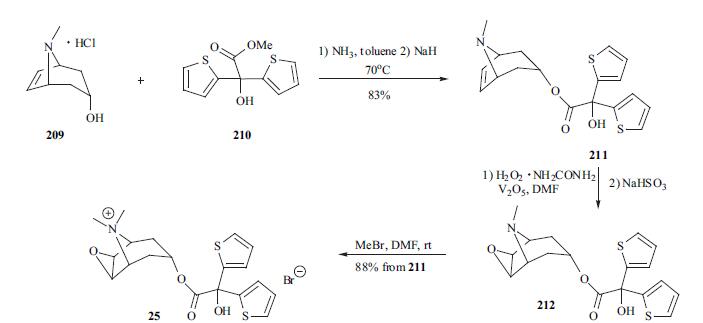
At least two synthetic paths have been disclosed in the patent and literature. The synthesis of tiotropium is depicted in the scheme. Tropenol hydrochloride 209 was first neutralized with ammonia in toluene and then the free base was reacted with methyl di-(2- thienyl)glycolate (210) in the presence of sodium hydride to furnish desired tropenol ester 211 in 83% yield. The vanadium-catalyzed oxidation of tropenol ester 211 using hydrogen peroxide-urea complex gave epoxide 212, which was converted into its quaternary salt 25 with methyl bromide. The last two steps were carried out in a one-pot process in 88%yield.
storage
Store at +4°C
Tiotropium bromide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 3010 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 10986 | 58 |
| Hebei Ganmiao New material Technology Co., LTD | +86-17332992504 +86-17332992504 | sales8@hbganmiao.com | China | 300 | 58 |
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 | sales@frappschem.com | China | 880 | 50 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 | FandaChem@Gmail.com | China | 9087 | 55 |
| Nanjing Finetech Chemical Co., Ltd. | 025-85710122 17714198479 | sales@fine-chemtech.com | CHINA | 885 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
View Lastest Price from Tiotropium bromide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-13 | Tiotropium bromide
136310-93-5
|
US $0.00-0.00 / g | 1g | 99% | 100kg/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-21 | Tiotropium bromide
136310-93-5
|
US $9.90 / KG | 1KG | 99% | 10 mt | Hebei Weibang Biotechnology Co., Ltd | |
 |
2024-11-19 | Tiotropium bromide
136310-93-5
|
US $45.00-77.00 / mg | 99.09% | 10g | TargetMol Chemicals Inc. |
-

- Tiotropium bromide
136310-93-5
- US $0.00-0.00 / g
- 99%
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Tiotropium bromide
136310-93-5
- US $9.90 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd
-

- Tiotropium bromide
136310-93-5
- US $45.00-77.00 / mg
- 99.09%
- TargetMol Chemicals Inc.
136310-93-5(Tiotropium bromide)Related Search:
1of4