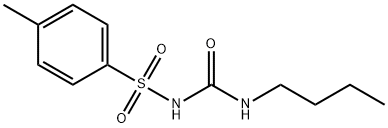
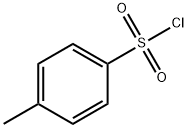
TOLBUTAMIDE
- CAS No.
- 64-77-7
- Chemical Name:
- TOLBUTAMIDE
- Synonyms
- Diaben;ORINASE;IRKG;d860;u2043;IRK16;GIRK4;BZ 55;D 860;tolbet
- CBNumber:
- CB4709039
- Molecular Formula:
- C12H18N2O3S
- Molecular Weight:
- 270.35
- MDL Number:
- MFCD00027169
- MOL File:
- 64-77-7.mol
- MSDS File:
- SDS
| Melting point | 128-130°C |
|---|---|
| Density | 1.2450 |
| refractive index | 1.6360 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Soluble in chloroform. |
| form | Solid |
| pka | pKa 5.32(H2O t = 37) (Uncertain) |
| color | White to Off-White |
| Water Solubility | 0.14g/L(25 ºC) |
| Merck | 14,9507 |
| BRN | 1984428 |
| Specific Activity | 50-60 mCi/mmol |
| Concentration | 0.1 mCi/ml |
| Solvent | Ethanol |
| Stability | Stable. Combustible. |
| CAS DataBase Reference | 64-77-7(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 982XCM1FOI |
| ATC code | A10BB03,V04CA01 |
| NCI Drug Dictionary | tolbutamide |
| EPA Substance Registry System | Tolbutamide (64-77-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H317 | |||||||||
| Precautionary statements | P261-P280g-P301+P312a-P321-P333+P313-P501a | |||||||||
| Hazard Codes | F,Xn | |||||||||
| Risk Statements | 20/21/22-40-43-36-11 | |||||||||
| Safety Statements | 26-36/37/39-36/37-16-24/25 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | YS4550000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29350090 | |||||||||
| Toxicity | LD50 oral in rat: 2490mg/kg | |||||||||
| NFPA 704 |
|
TOLBUTAMIDE price More Price(28)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR3251 | Tolbutamide pharmaceutical secondary standard, certified reference material | 64-77-7 | 500MG | $173 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1670003 | Tolbutamide United States Pharmacopeia (USP) Reference Standard | 64-77-7 | 200mg | $412 | 2024-03-01 | Buy |
| Sigma-Aldrich | 46968 | Tolbutamide VETRANAL?, analytical standard | 64-77-7 | 250mg | $53.6 | 2022-05-15 | Buy |
| TCI Chemical | T3690 | Tolbutamide >98.0%(HPLC)(T) | 64-77-7 | 1g | $26 | 2024-03-01 | Buy |
| Alfa Aesar | B21698 | Tolbutamide, 98% | 64-77-7 | 10g | $48.65 | 2024-03-01 | Buy |
TOLBUTAMIDE Chemical Properties,Uses,Production
Chemical Properties
White or almost white, crystalline powder.
Originator
Dolipol,Hoechst,France,1956
Uses
An antidiabetic, used as a hypoglycemic agent in veterinary medicine.
Uses
Tolbutamide, have been used in a cDNA microarray assay to probe changes in gene expression in HepG2 cells upon their administration. It has been utilized to counteract insulin activity in a patch-clamp investigation of ATP sensitive K+ channels in mouse pancreatic β-cells. The activity of various biotransformation enzymes in cultured primary rat proximal tubular cells in the presence of tolbutamide and other compounds has been studied.
Uses
It is used for type II diabetes mellitus of medium severity with no expressed microvascular complications.
Definition
ChEBI: An N-sulfonylurea that consists of 1-butylurea having a tosyl group attached at the 3-position.
Manufacturing Process
50 grams of n-butyl isocyanate are stirred at room temperature into a
suspension of 96 grams of sodium 4-methyl-benzenesulfonamide in 120 cc of
dry nitrobenzene and the whole is then heated for 7 hours at 100°C. After
being cooled, the reaction mixture, which is a thick magma, is diluted with
methylene chloride or ethyl acetate and the sodium salt of the sulfonylurea
formed is separated by centrifuging. The centrifuged crystalline residue freed
from organic solvents is dissolved in 500 to 600 cc of water heated at 50°C
and decolorized with animal charcoal.
The precipitate obtained by acidification with dilute hydrochloric acid is
dissolved in an equivalent quantity of dilute ammonia solution (about 1:20),
again treated with animal charcoal and reprecipitated with dilute hydrochloric
acid. In this manner N-4-methylbenzenesulfonyl-N'-n-butyl-urea is obtained in
analytically pure form in a yield of 70 to 80% of theory. It melts at 125° to
127°C (with decomposition).
Therapeutic Function
Oral hypoglycemic
General Description
White crystals.
General Description
Tolbutamide, 1-butyl-3-(p-tolylsulfonyl)urea (Orinase), occurs as a white, crystalline powderthat is insoluble in water and soluble in alcohol or aqueousalkali. It is stable in air.
Tolbutamide is absorbed rapidly in responsive diabetic patients.The blood sugar level reaches a minimum after 5 to8 hours. It is oxidized rapidly in vivo to 1-butyl-3-(p-carboxyphenyl)sulfonylurea, which is inactive. The metabolite isfreely soluble at urinary pH; if the urine is strongly acidified,however, as in the use of sulfosalicylic acid as a protein precipitant,a white precipitate of the free acid may be formed.
General Description
Tolbutamide is N-[(butylamino)carbonyl]-4-methylbenzenesulfonamide;or 1-butyl-3-(p-tolylsulfonyl)urea (Orinase,generic). Orinase Diagnostic was the sodium salt, which isfreely soluble in water for injection, but this product was discontinuedc. 2000.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
TOLBUTAMIDE is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). TOLBUTAMIDE is incompatible with acids. .
Fire Hazard
Flash point data for TOLBUTAMIDE are not available. TOLBUTAMIDE is probably combustible.
Biological Activity
tolbutamide is a potent inhibitor of camp with an ic50 value of 4mm [1].tolbutamide has been reported to inhibit both the basal and the cyclic amp-stimulated protein kinase activites with an ic50 value of 4mm for cyclic amp-dependent kinase activity. in addition, tolbutamide has been revealed to inhibit both soluble and membrane-bound protein kinase from canine heart. moreover, the tolbutamide inhibition of adipose tissue cyclic amp- dependent protein kinase is explanation for antilipolytic effects [1]. besides, tolbutamide and dbcamp has been exhibited to increase about four-fold levels of cx43 mrna and decrease about 80% the expression of ki-67 [2].
Mechanism of action
Tolbutamide is one of the most widely used antidiabetic agents. Its action is preferably connected with stimulatory action of β-cells in the pancreas, which results in intensive insulin secretion.
Clinical Use
Tolbutamide should be used only when the diabetic patientis an adult or shows adult-onset diabetes, and the patientshould adhere to dietary restrictions.
Safety Profile
Moderately toxic by ingestion and several other routes. A human teratogen. Human reproductive effects by ingestion and possibly other routes: stillbirth, developmental abnormalities of the cardlovascular (circulatory) system and urogenital system, and unspecified neonatal effects. Human systemic effects by ingestion: nausea or vomiting, hypoglycemia. Other experimental teratogenic and reproductive effects. Mutation data reported. Implicated in aplastic anemia. When heated to decomposition it emits very toxic fumes of NO, and SOx.
Synthesis
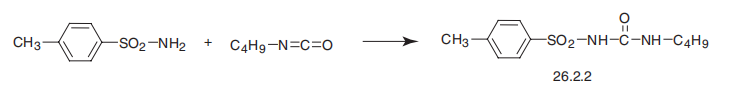
Tolbutamide, 1-butyl-3-p-toluenesulfonylurea (26.2.2), is made in a single step reaction by interaction of p-toluenesulfonylamide (in the form of sodium salt) with butylisocyanate.
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: effects enhanced by NSAIDs - avoid
with azapropazone.
Antibacterials: effects enhanced by chloramphenicol,
sulphonamides, tetracyclines and trimethoprim;
effect reduced by rifamycins.
Anticoagulants: effect possibly enhanced by
coumarins; also possibly changes to INR.
Antifungals: concentration increased by fluconazole
and miconazole, and possibly voriconazole.
Lipid-regulating drugs: possibly additive
hypoglycaemic effect with fibrates.
Sulfinpyrazone: enhanced effect of sulphonylureas.
Metabolism
Tolbutamide is metabolised in the liver by hydroxylation mediated by the cytochrome P450 isoenzyme CYP2C9. It is excreted in the urine chiefly as inactive metabolites.
References
[1] wray hl, harris aw. adenosine 3', 5'-monophosphate-dependent protein kinase in adipose tissue: inhibition by tolbutamide. biochem biophys res commun. 1973 jul 2;53(1):291-4.
[2] sánchez-alvarez r1, paíno t, herrero-gonzález s, medina jm, tabernero a. tolbutamide reduces glioma cell proliferation by increasing connexin43, which promotes the up-regulation of p21 and p27 and subsequent changes in retinoblastoma phosphorylation. glia. 2006 aug 1;54(2):125-34.
TOLBUTAMIDE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21637 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29885 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2930 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9636 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32072 | 58 |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | sales@afinechem.com | China | 15356 | 58 |
View Lastest Price from TOLBUTAMIDE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-09 | Tolbutamide
64-77-7
|
US $41.00 / mg | 99.65% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-07 | Tolbutamide
64-77-7
|
US $41.00 / mg | 99.65% | 10g | TargetMol Chemicals Inc. | ||
 |
2021-07-13 | Tolbutamide
64-77-7
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Tolbutamide
64-77-7
- US $41.00 / mg
- 99.65%
- TargetMol Chemicals Inc.
-

- Tolbutamide
64-77-7
- US $41.00 / mg
- 99.65%
- TargetMol Chemicals Inc.
-

- Tolbutamide
64-77-7
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd