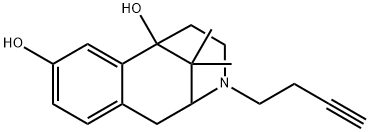
Butinazocine
- CAS No.
- 93821-75-1
- Chemical Name:
- Butinazocine
- Synonyms
- Butinazocine;2,6-Methano-3-benzazocine-6,8(1H)-diol, 3-(3-butyn-1-yl)-2,3,4,5-tetrahydro-11,11-dimethyl-
- CBNumber:
- CB51178192
- Molecular Formula:
- C18H23NO2
- Molecular Weight:
- 285.38
- MDL Number:
- MOL File:
- 93821-75-1.mol
| Boiling point | 456.1±45.0 °C(Predicted) |
|---|---|
| Density | 1.167±0.06 g/cm3(Predicted) |
| pka | 9.94±0.60(Predicted) |
| FDA UNII | W11ET455ZI |
Butinazocine Chemical Properties,Uses,Production
Originator
Butinazocine,ZYF Pharm Chemical
Manufacturing Process
A mixture of 59.5 g (0.2 mol) 2-(4-methoxybenzyl)-1,3,3-trimethyl-4-
piperidone hydrochloride and 53.8 g (0.4 mol) of aluminum trichloride and
54.0 g of nitrobenzene in 1500 ml of dry benzene are boiled under reflux for 1
h. After cooling the reaction mixture is extracted with 750 ml 4 N sodium
hydroxide solution, the temperature being maintained below 35°C. The
organic phase is separated and extracted with 750 ml 1 N hydrochloric acid.
The acid aequeous phase is rendered alkali by the addition of 100 ml 25%
ammonia and extracted three times with 250 ml chloroform. The collected
chloroformic phases are dried with sodium sulfate and evaporated under
reduced pressure. The residue, 46.7 g, is converted into the hydrochloride by
reaction with iso-propanol/HCl and crystallized from a mixture of methanol
and ethylacetate. 44.6 g of the 5-hydroxy-2’-methoxy-2,9,9-trimethyl-6,7-
benzomorphan hydrochloride are obtained, melting point 233-236° C (dec.).21.8 g (0.5 mol) of a 55% dispersion of sodium hydride in oil are added to
52.2 g (0.2 mol) of 5-hydroxy-2’-methoxy-2,9,9-trimethyl-6,7-benzomorphan
in 500 ml of dry peroxide-free tetrahydrofuran, followed by the drop-wise
addition over 45 min of 142.0 g (1.0 mol) of methyliodide, and the mixture is
stirred for 4 h at room temperature, 9 ml of water are added carefully to the
obtained reaction mixture and the tetrahydrofuran is evaporated off under
reduced pressure. After addition of 250 ml of water the residue is extracted 3
times with 250 ml of chloroform. The combined chloroformic phases are dried
over sodium sulfate and concentrated under reduced pressure. The residue is
converted into the hydrochloride, washed with toluene to remove paraffin oil
and crystallized form methanol/ethylacetate. There are obtained 53.5 g of the
2’,5-dimethoxy-2,9,9-trimethoxy-6,7-benzomorphan hydrochloride, melting
point 212-214°C (dec.).
A solution of 4.68 g (44 mmol) cyanogenbromide in 28 ml chloroform are
added drop-wise within 5 min to a solution of 8.25 g (30 mmol) 2’,5-
dimethoxy-2,9,9-trimethyl-6,7-benzomorphan in 20 ml dry ethanol free
chloroform. After boiling for 4 h under reflux the solution is concentrated
under reduced pressure, the residue dissolved in 150 ml toluene, washed
twice with 50 ml 2 N hydrochloric acid and once with water, dried over sodium
sulfate and evaporated to dryness under reduced pressure to yield 7.82 g 2-
cyano-2’,5-dimethoxy-2,9,9-trimethyl-6,7-benzomorphan. The obtained
residue is dissolved in 40 ml of dry peroxide-free tetrahydrofuran and the
solution added drop-wise under a nitrogen atmosphere over a period of 20
min to a suspension of 2.10 g (61.7 mmol) of lithium aluminum hydride in 85
ml tetrahydrofuran. After boiling for 3 h under reflux and cooling, 2.1 ml
water, 1.6 ml 4 N sodium hydroxide solution, 7.3 ml water and 85 ml
chloroform are added sequentially. After stirring for 30 min the obtained
hydroxide is filtered off over hyflo. The precipitate is stirred 3 times with 50
ml chloroform/butanol (9:1). The filtrate washed with water, dried over
sodium sulfate and concentrated under reduced pressure. The residue is
converted into the hydrochloride and crystallized from methanol/ethylacetate
to yield 4.9 g of the 2’,5-dimethoxy-9,9-dimethyl-6,7-benzomorphan
hydrochloride, melting point 220-222°C (dec.).
A solution of ethanethiol in dimethylformamide (DMF) are added dropwise to a
suspension of sodium hydride (55% suspension in oil) in dry DMF. The
obtained suspension is stirred for a further 45 min and a solution of 2’,5-
dimethoxy-9,9-dimethyl-6,7-benzomorphan in 190 ml of dry DMF are added
dropwise over 20 min. The initially formed volatile components are distilled off
and the reaction mixture heated until the DMF boils. After boiling for 6 h
under reflux the reaction mixture is concentrated under reduced pressure and
the residue taken up in toluene and 2 N hydrochloric acid. The acid aqueous
phase is made alkaline with 25% ammonia and extracted 3 times with
chloroform/butanol (8:2). After evaporation of the organic phase, the residue
is converted into the 9,9-dimethyl-2’,5-dihydroxy-6,7-benzomorphan
hydrochloride, (crystallized from isopropanol).
A mixture of 9,9-dimethyl-2’,5-dihydroxy-6,7-benzomorphan hydrochloride,
calcium carbonate and toluene-4-sulfonic acid but-3-ynyl ester in
dimethylformamide is heated and subsequently concentrated under reduced
pressure. The residue is taken up in a mixture of water and chloroform (1:1)
and the aequeous phase extracted twice with chloroform/butanol (9:1). The
combined organic phases are concentrated under reduced pressure to give the 2-butynyl-9,9-dimethyl-2’,5-dihydroxy-6,7-benzomorphan (crystallized from
methanol/ethylacetate).
Therapeutic Function
Analgesic
Butinazocine Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | sales@molcore.com | China | 49739 | 58 |
| Supplier | Advantage |
|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | 58 |