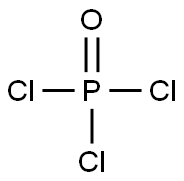
Phosphorus oxychloride
- CAS No.
- 10025-87-3
- Chemical Name:
- Phosphorus oxychloride
- Synonyms
- POCl3;PHOSPHORYL CHLORIDE;PHOSPHOROUS OXYCHLORIDE;PHOSPHORYL TRICHLORIDE;oxychloride;PHOSPHORUS(V) OXYCHLORIDE;PHOSPHOROXYCHLORIDE;phosphoric trichloride;Phosphoryl oxychloride;OPCl3
- CBNumber:
- CB5218244
- Molecular Formula:
- Cl3OP
Lewis structure

- Molecular Weight:
- 153.33
- MDL Number:
- MFCD00011443
- MOL File:
- 10025-87-3.mol
| Melting point | 1.25 °C(lit.) |
|---|---|
| Boiling point | 107 °C |
| Density | 1.645 g/mL at 25 °C(lit.) |
| vapor density | 5.3 (vs air) |
| vapor pressure | 104 mm Hg ( 50 °C) |
| refractive index |
n |
| Flash point | 105.8°C |
| storage temp. | Store below +30°C. |
| solubility | Phosphorus(V) oxychloride is soluble in many organic solvents. |
| form | Liquid |
| Specific Gravity | 1.692 (15/15℃) |
| color | Colorless |
| Odor | Pungent odour |
| PH | 1.0 (5g/l, H2O, 25℃) |
| Water Solubility | reacts exothermically |
| Sensitive | Moisture Sensitive |
| Merck | 14,7349 |
| Exposure limits | TLV-TWA0.628 mg/m3 (0.1 ppm)(ACGIH). |
| Dielectric constant | 14.0(22℃) |
| Stability | Stable. Reacts violently with water. Incompatible with many metals, alcohols, amines, phenol, DMSO, strong bases. |
| InChIKey | XHXFXVLFKHQFAL-UHFFFAOYSA-N |
| LogP | 0.357 (est) |
| Substances Added to Food (formerly EAFUS) | PHOSPHORUS OXYCHLORIDE |
| CAS DataBase Reference | 10025-87-3(CAS DataBase Reference) |
| EWG's Food Scores | 3 |
| FDA UNII | 9XM78OL22K |
| NIST Chemistry Reference | Phosphoryl chloride(10025-87-3) |
| EPA Substance Registry System | Phosphorus oxychloride (10025-87-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H314-H330-H372 | |||||||||
| Precautionary statements | P280-P301+P312-P303+P361+P353-P304+P340+P310-P305+P351+P338-P314 | |||||||||
| Hazard Codes | T+,C | |||||||||
| Risk Statements | 14-22-26-29-35-48/23-25 | |||||||||
| Safety Statements | 26-45-7/8-36/37/39 | |||||||||
| RIDADR | UN 1810 8/PG 2 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.1 ppm (0.6 mg/m3), STEL: 0.5 ppm (3 mg/m3) | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | TH4897000 | |||||||||
| F | 19-21 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28121020 | |||||||||
| Hazardous Substances Data | 10025-87-3(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
Phosphorus oxychloride price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.22339 | Phosphoryl chloride for synthesis | 10025-87-3 | 250ML | $96.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.22339 | Phosphoryl chloride for synthesis | 10025-87-3 | 1L | $171 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.22339 | Phosphoryl chloride for synthesis | 10025-87-3 | 300KG | $11630 | 2024-03-01 | Buy |
| Sigma-Aldrich | 201170 | Phosphorus(V) oxychloride 99% | 10025-87-3 | 5g | $45.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 201170 | Phosphorus(V) oxychloride 99% | 10025-87-3 | 1kg | $105 | 2024-03-01 | Buy |
Phosphorus oxychloride Chemical Properties,Uses,Production
Chemical Characteristics
Phosphorus oxychloride (chemical formula: POCl3), is a type of industrial raw material. It is a colorless and transparent liquid, and it has an unpleasant irritating odor. It will smoke intensely in humid air. Its relative density is 1.68, melting point is 1.25℃, boiling point is 105.1℃. It breaks down into phosphoric acid and hydrogen chloride in water and ethanol. When suddenly combined with a large amount of water, an intense reaction may occur. POCl3 reacts with water and alcohol to create phosphoric acid or phosphate. If alcohol replaces the water in the reaction, the end product will be trialkyl phosphate. This type of reaction often occurs in pyridine or ammonia, as it absorbs the produced HCl to stimulate the reaction. When catalyzed by a Lewis acid such as manganese chloride, POCl3 and a large amount of phenol (ArOH) heats to produce triaryl phosphate, such as in the reaction below: 3 C6H5OH + O=PCl3 → O=P(OC6H5)3 + 3 HCl. Phosphorus oxychloride is a Lewis base, and it produces compounds with many Lewis acids, such as in its reaction with titanium tetrachloride: Cl3P5+O− + TiCl4 → Cl3P5+O-−TiCl4. Its adduct with aluminum chloride (POCl3•AlCl3) is very stable, and thus POCl3 is used to remove the AlCl3 in the end products of Friedel-Crafts reactions. In the presence of AlCl3, POCl3 reacts with hydrogen bromide to create POBr3.
Usage
Phosphorus oxychloride can be used as a semiconductor dopant, and it is a raw material for light-conducting fibers. It is widely used in pesticides, pharmaceuticals, dyes, phosphates and flame retardant production. It is a raw material for producing organic phosphorus herbicide and chlordimeform, and it is a plasticizer in plastic production. Phosphorus oxychloride is also used in the chlorination of long-acting sulfa drugs, is an intermediate in dye production and a catalyst in organic synthesis of chlorinating agents, and it is an extracting agent in uranium mining. It is also used in producing pharmaceuticals.
Toxicology
Toxicity is similar to phosphorus trichloride, phosphorus pentachloride and phosgene. Large mice, oral, LD50: 380 mg/kg; inhaled, LC50: 32 ppm/4H. Acute poisoning in small mice results in restlessness, upper respiratory tract and conjunctival irritation, depression, convulsions, unsteady walking, lying on the side, and eventually, death. For large mice, in addition to the symptoms above, also exhibited tearing, cornea clouding, and pulmonary edema. Subacute and chronic toxicity: large mice, inhaled for 60 days at a concentration of 33.5mg/m3, exhibited slowed weight gain, skin ulcers, decreases survival rates in lung macrophages, no liver and kidney functions, and organ characteristic changes.
Hazards & Safety Information
Category Corrosive items
Toxicity grading highly toxic
Acute Toxicity Oral-Rat LD50: 380 mg/kg
Flammability and Hazardous characteristics being explosive upon coming across water with release of toxic chloride, phosphorus oxide gas
Storage and transportation characteristics Ventilated, low temperature and dry; and store it separately from alkali
Fire Extinguishing agent dry sand, dry stone powder; prohibit the usage of water
Occupational Standard TLV-TWA 0.1 PPM (0.6 mg/m3); STEL 0.5 PPM (3 mg/m3)
Chemical Properties
Phosphorus oxychloride is a clear, colorless to yellow, fuming, oily liquid with a pungent and musty odor.
Physical properties
Colorless fuming liquid with a pungent odor; density 1.645 g/mL; freezes at 1°C; boils at 105.5°C; reacts with water and ethanol.
Uses
Phosphorus oxychloride is used to produce hydraulic fluids, plasticizers, and fireretarding agents; as a chlorinating agent; and as a solvent in cryoscopy.
Uses
Phosphorus oxychloride is an important intermediate in the production of triarylphosphate esters (e.g., triphenyl phosphate and tricresyl phosphate), which have been used as flame retardants and plasticizers for PVC. It is acutely toxic to the eyes, throat, and respiratory tract. Phosphorus oxychloride is also used in nuclear reprocessing, as chlorinating agent, especially to replace oxygen in organic compounds, as solvent in cryoscopy and the semiconductor industry.
Preparation
Phosphorus oxychloride can be prepared from phosphorus trichloride or phosphorus pentachloride. It can be obtained from phosphorus trichloride by cautious addition of potassium chlorate:3PCl3 + KClO3 → 3POCl3 + KCl The oxychloride also is obtained by the action of boric acid or oxalic acid with phosphorus pentachloride: 3PCl5 + 2B(OH)3 → 3POCl3 + B2O3 + 6HCl PCl5 + (COOH)2 → POCl3 + CO + CO2 + 2HCl Phosphorus oxychloride also is made by heating calcium phosphate in a current of chlorine and carbon monoxide at 350°C: 2Ca3(PO4)2 + 9Cl2 + 6CO → 4POCl3 + 6CaCO3 Alternatively, heating a mixture of calcium phosphate and carbon in a current of chlorine at 750°C yields the oxychloride.
Definition
A white crystalline solid. It is a monobasic acid forming the anion H2PO2 – in water. The sodium salt, and hence the acid, can be prepared by heating yellow phosphorus with sodium hydroxide solution. The free acid and its salts are powerful reducing agents.
Reactivity Profile
Phosphorus oxychloride is water reactive. Incompatible with strong oxidizing agents, alcohols, bases (including amines). May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291]. Combining the chloride with zinc dust caused immediate ignition, due to the formation of phosphine gas which ignites, [Mellor, 1940, Vol. 8, 1025]. An exotherm starting with the mixing of Phosphorus oxychloride with acetone (a ketone) lead to an explosion, may behave similarly with other ketones, [Organic Process Research and Development, Vol.4, No. 6,200, "Phosphorus oxychloride and Acetone: An Incompatibility Investigation Using ARC."]
Hazard
The compound is highly irritating to skin, eyes and mucous membranes. Inhaling its vapors can cause pulmonary edema.
Health Hazard
Inhalation of vapors of phosphorus oxychloride produced acute and chronic toxicity in test subjects. In humans, exposure to its vapors may cause headache, dizziness, weakness, nausea, vomiting, coughing, chest pain, bronchitis, and pulmonary edema. Most of these symptoms are manifested from chronic exposure to its vapors.
LC50 value, inhalation (rats): 48 ppm (301 mg/m3)/4 h
Vapors of this compound are an irritant to the eyes and mucous membranes. The liquidis corrosive and can cause skin burns. An oral LD50 value for rats is documented to be 380 mg/kg (NIOSH 1986)..
Fire Hazard
Poisonous, corrosive, and irritating gases are generated when Phosphorus oxychloride is heated or is in contact with water. Phosphorus oxychloride may ignite other combustible materials (wood, paper, oil, etc.). Phosphorus oxychloride reacts violently with water. When heated to decomposition, Phosphorus oxychloride emits toxic fumes of chlorides and oxides of phosphorus; Phosphorus oxychloride will react with water or steam to produce heat and toxic and corrosive fumes. Incompatible with carbon disulfide; N,N-dimethylformamide; 2,5-dimethylpyrrole; 2,6-dimethyl- pyridine N-oxide; dimethylsulfoxide; Ferrocene-1,1-dicarboxylic acid; water; and zinc. Do not store with combustible materials, particularly fibrous organic materials, or with electrical or other equipment that can be corroded. Reacts violently with moisture.
Potential Exposure
Phosphorus oxychloride is used in the manufacture of pesticides, pharmaceuticals, plasticizers, gasoline additives; and hydraulic fluids.
Shipping
UN1810 Phosphorus oxychloride, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material, Hazard Zone B.
Purification Methods
Distil the liquid under reduced pressure to separate it from the bulk of the HCl and the phosphoric acid (from hydrolysis); the middle fraction is re-distilled into ampoules containing a little purified mercury. These ampoules are sealed and stored in the dark for 4-6weeks with occasional shaking to facilitate reaction of any free chloride with the mercury. The POCl3 is then again fractionally distilled and stored in sealed ampoules in the dark until required [Herber J Am Chem Soc 82 792 1960]. Lewis and Sowerby [J Chem Soc 336 1957] refluxed their distilled POCl3 with Na wire for 4hours, then removed the Na and again distilled. Use Na only with almost pure POCl3 to avoid explosions. HARMFUL VAPOURS; work in an efficient fume cupboard.
Incompatibilities
A powerful oxidizer. Violently decomposes in water, forming heat and hydrochloric and phosphoric acids. Violent reaction with alcohols, phenols, amines, reducing agents; combustible materials; carbon disulfide; dimethylformamide, and many other many materials. Rapid corrosion of metals, except nickel and lead.
Waste Disposal
Pour onto sodium bicarbonate. Spray with aqueous ammonia and add crushed ice. Neutralize and pour into drain with running water. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Phosphorus oxychloride Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 | admin@firsky-cn.com | China | 427 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20285 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2930 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| Richest Group Ltd | 18017061086 | oled@richest-group.com | CHINA | 5600 | 58 |
Related articles
- Phosphorus oxychloride:Reactions with water,Uses,Hazards and Warnings
- Phosphorus oxychloride is a colourless liquid with the formula POCl3.
- Nov 11,2024
- Uses and preparation of Phosphorus oxychloride
- Phosphorus oxychloride is an inorganic compound which is commonly referred to as phosphorous oxychloride or as phosphoryl chlo....
- Jul 18,2022
View Lastest Price from Phosphorus oxychloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-04 | Phosphorus oxychloride
10025-87-3
|
US $10.00 / KG | 1KG | 99% | 100 mt | Hebei Weibang Biotechnology Co., Ltd | |
 |
2023-12-23 | Phosphorus oxychloride
10025-87-3
|
US $50.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-10-09 | Phosphoryl trichloride
10025-87-3
|
US $30.00 / KG | 1KG | 99% | 20T | Firsky International Trade (Wuhan) Co., Ltd |
-

- Phosphorus oxychloride
10025-87-3
- US $10.00 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd
-

- Phosphorus oxychloride
10025-87-3
- US $50.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- Phosphoryl trichloride
10025-87-3
- US $30.00 / KG
- 99%
- Firsky International Trade (Wuhan) Co., Ltd
10025-87-3(Phosphorus oxychloride)Related Search:
1of4