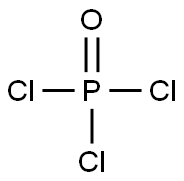
포스포러스옥시클로라이드
|
|
포스포러스옥시클로라이드 속성
- 녹는점
- 1.25 °C(lit.)
- 끓는 점
- 107 °C
- 밀도
- 1.645 g/mL at 25 °C(lit.)
- 증기 밀도
- 5.3 (vs air)
- 증기압
- 104 mm Hg ( 50 °C)
- 굴절률
- n
20/D 1.461
- 인화점
- 105.8°C
- 저장 조건
- Store below +30°C.
- 용해도
- 인(V) 옥시염화물은 많은 유기 용매에 용해됩니다.
- 물리적 상태
- 액체
- Specific Gravity
- 1.692 (15/15℃)
- 색상
- 무색의
- 수소이온지수(pH)
- 1.0 (5g/l, H2O, 25℃)
- 냄새
- 매운 냄새
- 수용성
- 발열 반응
- 감도
- Moisture Sensitive
- Merck
- 14,7349
- 노출 한도
- TLV-TWA0.628 mg/m3 (0.1 ppm)(ACGIH).
- Dielectric constant
- 14.0(22℃)
- 안정성
- 안정적인. 물과 격렬하게 반응함. 많은 금속, 알코올, 아민, 페놀, DMSO, 강염기와 호환되지 않습니다.
- InChIKey
- XHXFXVLFKHQFAL-UHFFFAOYSA-N
- LogP
- 0.357 (est)
- CAS 데이터베이스
- 10025-87-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T+,C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 14-22-26-29-35-48/23-25 | ||
| 안전지침서 | 26-45-7/8-36/37/39 | ||
| OEB | C | ||
| OEL | TWA: 0.1 ppm (0.6 mg/m3), STEL: 0.5 ppm (3 mg/m3) | ||
| 유엔번호(UN No.) | UN 1810 8/PG 2 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | TH4897000 | ||
| F 고인화성물질 | 19-21 | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | II | ||
| HS 번호 | 28121020 | ||
| 유해 물질 데이터 | 10025-87-3(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-28728 | ||
| 유해화학물질 필터링 | 97-1-218 | ||
| 중점관리물질 필터링 | 별표1-143 | ||
| 사고대비 물질 필터링 | 54 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 옥시염화 인 및 이를 25% 이상 함유한 혼합물 |
포스포러스옥시클로라이드 C화학적 특성, 용도, 생산
물성
안정적. 물과 격렬하게 반응합니다. 많은 금속, 알콜, 아민, 페놀, DMSO, 강염기와는 호환되지 않습니다.용도
디 페닐 이소 옥틸 포스페이트 및 트리 에틸 포스페이트, 플라스틱 가소제, 유기 인계 농약 및 장시간 작용하는 술폰 아미드와 같은 포스 파 시드의 제조에 사용됩니다. 또한 염료 중간체, 염소화 제 및 촉매, 난연제의 유기 합성으로 사용할 수 있습니다. 태양 에너지 산업, 집적 회로, 분리 장치, 조립식 막대 및 기타 액체 인 공급원에서 전자 등급 tricloxane을 사용하여 인산염을 제조 할 수도 있습니다. 반도체 산업에서 주로 N 형 도핑 소스에 사용되며 0 ℃에서 반응 효과가 가장 좋습니다.화학적 성질
Phosphorus oxychloride is a clear, colorless to yellow, fuming, oily liquid with a pungent and musty odor.물리적 성질
Colorless fuming liquid with a pungent odor; density 1.645 g/mL; freezes at 1°C; boils at 105.5°C; reacts with water and ethanol.용도
Phosphorus oxychloride is an important intermediate in the production of triarylphosphate esters (e.g., triphenyl phosphate and tricresyl phosphate), which have been used as flame retardants and plasticizers for PVC. It is acutely toxic to the eyes, throat, and respiratory tract. Phosphorus oxychloride is also used in nuclear reprocessing, as chlorinating agent, especially to replace oxygen in organic compounds, as solvent in cryoscopy and the semiconductor industry.제조 방법
Phosphorus oxychloride can be prepared from phosphorus trichloride or phosphorus pentachloride. It can be obtained from phosphorus trichloride by cautious addition of potassium chlorate:3PCl3 + KClO3 → 3POCl3 + KCl The oxychloride also is obtained by the action of boric acid or oxalic acid with phosphorus pentachloride: 3PCl5 + 2B(OH)3 → 3POCl3 + B2O3 + 6HCl PCl5 + (COOH)2 → POCl3 + CO + CO2 + 2HCl Phosphorus oxychloride also is made by heating calcium phosphate in a current of chlorine and carbon monoxide at 350°C: 2Ca3(PO4)2 + 9Cl2 + 6CO → 4POCl3 + 6CaCO3 Alternatively, heating a mixture of calcium phosphate and carbon in a current of chlorine at 750°C yields the oxychloride.정의
A white crystalline solid. It is a monobasic acid forming the anion H2PO2 – in water. The sodium salt, and hence the acid, can be prepared by heating yellow phosphorus with sodium hydroxide solution. The free acid and its salts are powerful reducing agents.일반 설명
A colorless fuming liquid with a pungent odor. Density 14.0 lb / gal. Very toxic by inhalation and corrosive to metals and tissue. Used in gasoline additives and hydraulic fluids.반응 프로필
Phosphorus oxychloride is water reactive. Incompatible with strong oxidizing agents, alcohols, bases (including amines). May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291]. Combining the chloride with zinc dust caused immediate ignition, due to the formation of phosphine gas which ignites, [Mellor, 1940, Vol. 8, 1025]. An exotherm starting with the mixing of Phosphorus oxychloride with acetone (a ketone) lead to an explosion, may behave similarly with other ketones, [Organic Process Research and Development, Vol.4, No. 6,200, "Phosphorus oxychloride and Acetone: An Incompatibility Investigation Using ARC."]위험도
The compound is highly irritating to skin, eyes and mucous membranes. Inhaling its vapors can cause pulmonary edema.건강위험
Inhalation of vapors of phosphorus oxychloride produced acute and chronic toxicity in test subjects. In humans, exposure to its vapors may cause headache, dizziness, weakness, nausea, vomiting, coughing, chest pain, bronchitis, and pulmonary edema. Most of these symptoms are manifested from chronic exposure to its vapors.LC50 value, inhalation (rats): 48 ppm (301 mg/m3)/4 h
Vapors of this compound are an irritant to the eyes and mucous membranes. The liquidis corrosive and can cause skin burns. An oral LD50 value for rats is documented to be 380 mg/kg (NIOSH 1986)..
화재위험
Poisonous, corrosive, and irritating gases are generated when Phosphorus oxychloride is heated or is in contact with water. Phosphorus oxychloride may ignite other combustible materials (wood, paper, oil, etc.). Phosphorus oxychloride reacts violently with water. When heated to decomposition, Phosphorus oxychloride emits toxic fumes of chlorides and oxides of phosphorus; Phosphorus oxychloride will react with water or steam to produce heat and toxic and corrosive fumes. Incompatible with carbon disulfide; N,N-dimethylformamide; 2,5-dimethylpyrrole; 2,6-dimethyl- pyridine N-oxide; dimethylsulfoxide; Ferrocene-1,1-dicarboxylic acid; water; and zinc. Do not store with combustible materials, particularly fibrous organic materials, or with electrical or other equipment that can be corroded. Reacts violently with moisture.Safety Profile
Poison by inhalation and ingestion. A corrosive eye, skin, and mucous membrane irritant. Potentially explosive reaction with water evolves hydrogen chloride and phosphine, which then ignites. Explosive reaction with 2,6dimethylpyridine N-oxide, dimethyl sulfoxide, ferrocene1 ,l'-dicarboxylic acid, pyridne N-oxide (above bO'C), sodmm + heat. Violent reaction or ignition with BI3, carbon disulfide, 2,5-dimethyl pyrrole + dimethyl formamide, organic matter, zinc powder. Reacts with water or steam to produce heat and toxic and corrosive fumes. Incompatible with carbon disulfide, N,Ndimethyl-formamide, 2,5-dunethylpyrrole, 2,6-dimethylpyridine N-oxide, dimethylsulfoxide, ferrocene1 ,I-dicarboxylic acid, water, zinc. When heated to decomposition it emits highly toxic fumes of Cland POx잠재적 노출
Phosphorus oxychloride is used in the manufacture of pesticides, pharmaceuticals, plasticizers, gasoline additives; and hydraulic fluids.운송 방법
UN1810 Phosphorus oxychloride, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material, Hazard Zone B.Purification Methods
Distil the liquid under reduced pressure to separate it from the bulk of the HCl and the phosphoric acid (from hydrolysis); the middle fraction is re-distilled into ampoules containing a little purified mercury. These ampoules are sealed and stored in the dark for 4-6weeks with occasional shaking to facilitate reaction of any free chloride with the mercury. The POCl3 is then again fractionally distilled and stored in sealed ampoules in the dark until required [Herber J Am Chem Soc 82 792 1960]. Lewis and Sowerby [J Chem Soc 336 1957] refluxed their distilled POCl3 with Na wire for 4hours, then removed the Na and again distilled. Use Na only with almost pure POCl3 to avoid explosions. HARMFUL VAPOURS; work in an efficient fume cupboard.비 호환성
A powerful oxidizer. Violently decomposes in water, forming heat and hydrochloric and phosphoric acids. Violent reaction with alcohols, phenols, amines, reducing agents; combustible materials; carbon disulfide; dimethylformamide, and many other many materials. Rapid corrosion of metals, except nickel and lead.폐기물 처리
Pour onto sodium bicarbonate. Spray with aqueous ammonia and add crushed ice. Neutralize and pour into drain with running water. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.포스포러스옥시클로라이드 준비 용품 및 원자재
원자재
준비 용품
2,4-DICHLOROTHIENO[3,2-D]PYRIMIDINE
DICAPTHON
2,2'-비티오펜-5-카르복스알데하이드
5-METHYL-2-METHYLSULFANYL-PYRIMIDINE
벤잘데하이드질소머스타드
2-Trifluoromethylbenzylsulfonyl chloride
4,6-Dichloro-5-methoxypyrimidine
2-클로로-4-메틸퀴놀린-3-탄소니트릴
4-(4-(BIS-(2-CHLOROETHYL)AMINO)BENZYLIDENE-2-PHENYL-OXAZOLINE-5-ONE
HEXAFLUOROGLUTARIC ANHYDRIDE
6-N-헥실아미노퓨린
2,4-Dichlorothieno[3,2-d]pyrimidine
히드록시프로필 디스타치 인산염
2,6-디클로로-4,8-디피페리디노피리미디노[5,4-d]피리미딘
2,4-Dichloro-5-nitropyrimidine
P-니트로페닐 인산, 디나트륨 염, 헥사수화물
살리실산4-TERT-부틸페닐에스테르
트리옥틸 인산염
2-ETHOXY-1-NAPHTHALDEHYDE
클로르암부실
4-Chloro-7-azaindole
염기성청색7호
2-(클로로메틸)-5-프로폭시피리딘
Niclosamide
2-Chloro-4-methylpyrimidine
4-클로로-3,5-디니트로벤즈알데히드
6-CHLORO-5-CYANO-4-METHOXYMETHYL-3-NITRO-2-PICOLINE
2-클로로-3',4'-디하이드록시아세토페논
2,4,6-Trichloropyridine
다이스타치포스페이트
Quinazoline, 2-methyl- (6CI,7CI,8CI,9CI)
Acetylated Dishtarch Phosphate
Pyridine-3-sulfonyl chloride hydrochloride
4-(Dimethylamino)benzophenone
2-클로로-5-티오펜카르복스알데히드
P-톨릴-아세틸클로라이드
CHROMONE-3-CARBOXALDEHYDE
3-클로로-1H-인다졸
4-TRIFLUOROMETHYLBENZYLSULFONYL CHLORIDE
2-CHLOROLEPIDINE
포스포러스옥시클로라이드 공급 업체
글로벌( 403)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 |
admin@firsky-cn.com | China | 427 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20288 | 58 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 |
FandaChem@Gmail.com | China | 9220 | 55 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2930 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 |
abby@weibangbio.com | China | 8817 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 |
sales@amoychem.com | China | 6383 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49374 | 58 |