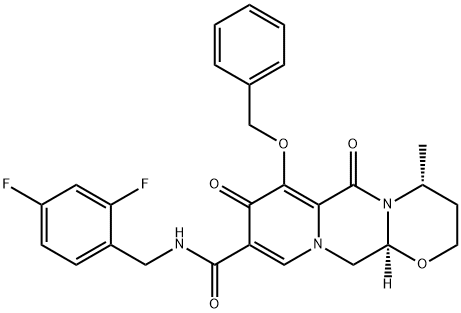
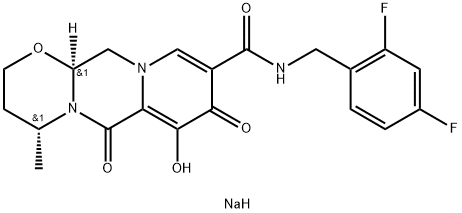
Dolutegravir sodium
- CAS No.
- 1051375-19-9
- Chemical Name:
- Dolutegravir sodium
- Synonyms
- DOLUTEGRAVIR SODIUM;Dolutegravi Sodium;Dolutegravir sodiuM salt;GSK1349572 sodiuM salt USP/EP/BP;Sodium (4R,12aS)-9-((2,4-difluorobenzyl)carbamoyl)-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazin-7-olate;158439;GSK 1349572A;Dulutevir sodium;Dulutewei sodium salt;GSK1349572 sodiuM salt
- CBNumber:
- CB52646573
- Molecular Formula:
- C20H20F2N3NaO5
- Molecular Weight:
- 443.38
- MDL Number:
- MFCD28405599
- MOL File:
- 1051375-19-9.mol
| Melting point | >300oC |
|---|---|
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly, Heated) |
| form | Solid |
| color | White to Green |
| Stability | Hygroscopic |
| InChIKey | FWLDGCYHMZPGGI-SBBUJZKLNA-N |
| SMILES | O=C1N2[C@@H](CCO[C@@]2([H])CN2C=C(C(=O)NCC3C=CC(F)=CC=3F)C(=O)C(O)=C12)C.[NaH] |&1:3,7,r| |
| FDA UNII | 1Q1V9V5WYQ |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H400-H410 |
| Precautionary statements | P273-P391-P501-P273-P391-P501 |
Dolutegravir sodium price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | D528805 | DolutegravirSodiumSalt | 1051375-19-9 | 25mg | $335 | 2021-12-16 | Buy |
| Usbiological | 447645 | Dolutegravir Sodium Salt | 1051375-19-9 | 2mg | $446 | 2021-12-16 | Buy |
| ApexBio Technology | B5856 | GSK1349572sodiuMsalt | 1051375-19-9 | 50mg | $585 | 2021-12-16 | Buy |
| ChemScene | CS-3496 | Dolutegravirsodium 99.96% | 1051375-19-9 | 2mg | $97 | 2021-12-16 | Buy |
| ApexBio Technology | B5856 | GSK1349572sodiuMsalt | 1051375-19-9 | 5mg | $110 | 2021-12-16 | Buy |
Dolutegravir sodium Chemical Properties,Uses,Production
Description
Dolutegravir, also known as DTG or dolutegravir sodium, is an antiretroviral therapy drug used to treat HIV infection. It belongs to the Integrase Strand Transfer inhibitor (INSTi) class of drugs and was fast-tracked by the FDA in February 2012. GlaxoSmithKline developed and markets dolutegravir sodium (Tivicay), which received FDA approval in August 2013 as a novel integrase inhibitor for HIV treatment, including adults undergoing their first treatment as well as those who have been treated with other integrase transfer strand inhibiting agents.
Uses
Dolutegravir, a second-generation HIV-1 integrase strand transfer inhibitor, is commonly used along with other medications to manage HIV infection. Its potency in inhibiting HIV replication has been demonstrated in various cell types infected with a self-inactivating PHIV lentiviral vector, including peripheral blood mononuclear cells (PBMCs), MT-4 cells, and CIP4 cells.
Definition
ChEBI: Dolutegravir sodium is an organic sodium salt that is the monosodium salt of dolutegravir. Used for treatment of HIV-1. It has a role as a HIV-1 integrase inhibitor. It contains a dolutegravir(1-).
Side effects
Dolutegravir, an HIV medication, can lead to a variety of side effects. While some can be serious, many, such as nausea or sporadic dizziness, can be effectively managed. Dolutegravir may also cause alterations in your immune system, resulting in a condition known as immune reconstitution inflammatory syndrome (IRIS).
clinicalinfo.hiv.gov/en/drugs/dolutegravir/patient
Synthesis
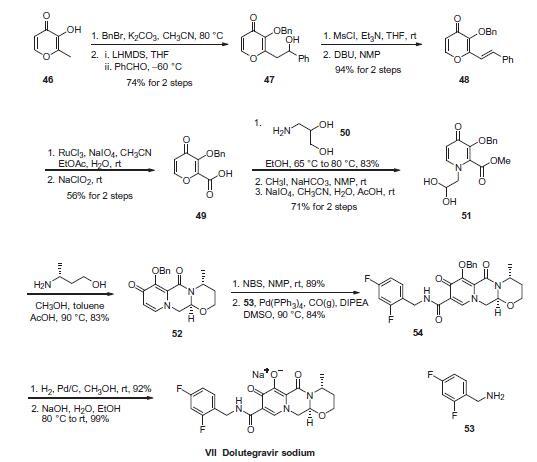
The most likely process-scale synthesis of dolutegravir sodium, began with benzyl protection and alkylation of pyrone 46 with benzaldehyde, yielding alcohol 47 in 74% over 2 steps. Alcohol mesylation and in situ elimination provided the styrenyl olefin 48 in 94% yield, which further underwent an oxidative cleavage of the olefin to generate 49 by sequential addition of RuCl3/NaIO4 and NaClO2 (56% overall yield). Treatment of pyranone 49 with 3-amino-propane-2-diol (50) in ethanol at elevated temperatures delivered the corresponding pyridinone in 83% yield, and this was followed by esterification and sodium periodate-mediated diol cleavage to furnish intermediate 51 in 71% overall yield across the two-step sequence. l Next, the key ring-forming step in the synthesis of dolutegravir sodium consisted of cyclization of 51 with (R)-3- amino-butan-1-ol, a process which relies on substrate control to provide the desired tricyclic carbamoylpyridone system 52 in high stereoselectivity (20/1 in favor of the desired isomer).51 Previously, cyclization of systems such as 51 with unsubstituted amino alcohols were found to yield a mixture of diastereomeric products, therefore indicating the pivotal role of the chiral amino alcohol in influencing stereochemical bias during the overall cyclization step. In practice, reaction of 51 with (R)-3-amino-butan-1-ol at 90 ?? led to isolation of a single cyclization product 52, after recrystallization from EtOAc. From 52, N-bromosuccinimide (NBS) bromination and subsequent treatment with amine 53 under palladium-catalyzed amidocarbonylative conditions led to amide 54 in 75% yield over 2 steps. Finally, removal of the benzyl group and subsequent crystallization using sodium hydroxide in water and ethanol provided dolutegravir sodium (VII) in 99% yield.
in vitro
gsk1349572 is a two-metal-binding hiv integrase strand transfer inhibitor whose mechanism of action was established through resistance passage experiments, integrase enzyme assays, mechanistic cellular assays and activity against viral strains resistant to other classes of anti-hiv agents. in a variety of cellular antiviral assays, gsk1349572 inhibited hiv replication with subnanomolar or low-nanomolar potency and with a selectivity index of 9,400. the protein-adjusted half-maximal effective concentration extrapolated to 100% human serum was 38 nm [1].
References
[1] kobayashi m, yoshinaga t, seki t, wakasa-morimoto c, brown kw, ferris r, foster sa, hazen rj, miki s, suyama-kagitani a, kawauchi-miki s, taishi t, kawasuji t, johns ba, underwood mr, garvey ep, sato a, fujiwara t. in vitro antiretroviral properties of s/gsk1349572, a next-generation hiv integrase inhibitor. antimicrob agents chemother. 2011 feb;55(2):813-21.
[2] van lunzen j, maggiolo f, arribas jr, rakhmanova a, yeni p, young b, rockstroh jk, almond s, song i, brothers c, min s. once daily dolutegravir (s/gsk1349572) in combination therapy in antiretroviral-naive adults with hiv: planned interim 48 week results from spring-1, a dose-ranging, randomised, phase 2b trial. lancet infect dis. 2012 feb;12(2):111-8.
Dolutegravir sodium Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Beijing Hope Pharmaceutical Co., Ltd. | +86-010-67886402 +8613611125266 | market@hopelife.cn | China | 71 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20287 | 58 |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 | sales@sjar-tech.com | China | 444 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18779 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29884 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Beijing Yibai Biotechnology Co., Ltd | 0086-182-6772-3597 | sales04@yibaibiotech.com | CHINA | 419 | 58 |
Related articles
- The Role of Dolutegravir Sodium in Transforming HIV Care: From Mechanism of Action to Clinical Application
- Dolutegravir sodium is a potent antiretroviral medication primarily used in the treatment of human immunodeficiency virus (HIV....
- Apr 8,2024
- Use of the anti-HIV drug (Dolutegravir sodium)
- Dolutegravir sodium is an oral, World Health Organisation-recommended first-line antiretroviral drug that belongs to a class ....
- Nov 10,2023
View Lastest Price from Dolutegravir sodium manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | Dolutegravir sodium
1051375-19-9
|
US $31.00-72.00 / mg | 99.74% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-18 | Dolutegravir Sodium
1051375-19-9
|
US $0.00 / g | 1g | More Than 99% | 50kg/Month | BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | |
 |
2024-03-23 | Dolutegravir sodium
1051375-19-9
|
US $6.00-0.50 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd |
-

- Dolutegravir sodium
1051375-19-9
- US $31.00-72.00 / mg
- 99.74%
- TargetMol Chemicals Inc.
-

- Dolutegravir Sodium
1051375-19-9
- US $0.00 / g
- More Than 99%
- BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
-

- Dolutegravir sodium
1051375-19-9
- US $6.00-0.50 / KG
- 99%
- Henan Fengda Chemical Co., Ltd





