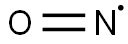NITRIC OXIDE
- CAS No.
- 10102-43-9
- Chemical Name:
- NITRIC OXIDE
- Synonyms
- NO;nitric;NITROGEN MONOXIDE;Nitrogen monooxide;DIBROMOHEXAMIDINE ISETHIONATE;Stickoxide;oxo-amidoge;nitricoxide0;NITRIC OXIDE;stickmonoxyd
- CBNumber:
- CB5433122
- Molecular Formula:
- NO*
Lewis structure

- Molecular Weight:
- 30.01
- MDL Number:
- MFCD00011525
- MOL File:
- 10102-43-9.mol
| Melting point | −163.6 °C(lit.) |
|---|---|
| Boiling point | −151.7 °C(lit.) |
| Density | d-150.2 (liq) 1.27; Relative d (gas) 1.036 (air = 1); Absolute d (gas) 1.227 (air = 1) |
| vapor density | 1.05 (vs air) |
| refractive index | nD25 1.0002697 |
| solubility | At 20 °C and at a pressure of 101 kPa, 1 volume dissolves in about 21 volumes of water. |
| form | colorless gas |
| color | colorless |
| Water Solubility | slightly soluble H2O [HAW93] |
| Merck | 13,6611 |
| Exposure limits | TLV-TWA 25 ppm (~30 mg/m3) (ACGIH, MSHA, and OSHA). |
| Stability | Spontaneously reacts with oxygen in air to yield brown nitrogen dioxide. Reacts violently or explosively with ammonia and many organic materials. |
| InChIKey | MWUXSHHQAYIFBG-UHFFFAOYSA-N |
| CAS DataBase Reference | 10102-43-9(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 31C4KY9ESH |
| ATC code | R07AX01 |
| EPA Substance Registry System | Nitric oxide (10102-43-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS03,GHS04,GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H270-H280-H314-H330 | |||||||||
| Precautionary statements | P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338-P410+P403 | |||||||||
| Hazard Codes | O,T | |||||||||
| Risk Statements | 8-23-34-44 | |||||||||
| Safety Statements | 17-23-36/37/39-45 | |||||||||
| RIDADR | UN 1660 2.3 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 25 ppm (30 mg/m3) | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | QX0525000 | |||||||||
| DOT Classification | 2.3, Hazard Zone A (Gas poisonous by inhalation) | |||||||||
| HazardClass | 2.3 | |||||||||
| Toxicity | LCLo inhalation in dog: 5000ppm/25M | |||||||||
| IDLA | 100 ppm | |||||||||
| NFPA 704 |
|
NITRIC OXIDE price More Price(10)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | NIST2737 | Nitric oxide in nitrogen NIST?SRM?2737,nominal500nmol/mol | 10102-43-9 | 1cylinder | $9930 | 2024-03-01 | Buy |
| Sigma-Aldrich | NIST2630 | Nitric oxide in nitrogen NIST?SRM?2630,nominal1500umol/mol | 10102-43-9 | 1ea | $3950 | 2024-03-01 | Buy |
| Sigma-Aldrich | NIST2738 | Nitric oxide in nitrogen NIST?SRM?2738,nominal1000nmol/mol | 10102-43-9 | 1cylinder | $9660 | 2023-06-20 | Buy |
| Sigma-Aldrich | 295566 | Nitric oxide 98.5% | 10102-43-9 | 17L | $721 | 2022-05-15 | Buy |
| Usbiological | 405810 | Nitric Oxide | 10102-43-9 | 1Kit | $479 | 2021-12-16 | Buy |
NITRIC OXIDE Chemical Properties,Uses,Production
Uses
Nitric oxide is an important intermediate in the production of nitric acid. Fluorine, chlorine, and bromine react with nitric oxide to form the corresponding nitrosyl halide. [t is also used in making mixtures for calibration standards for stationary and mobile exhaust emission measurements.
Description
near room temperature (its liquid density at 20°C is 1.45 g/cm3). Nitrogen monoxide (NO) is commonly called nitric oxide,Nitric oxide is colorless and has a sharp sweet odor;Nitric oxide is nonfl ammable, toxic gases.Nitric oxide is a free radical that quickly reacts in air to produce nitrogen dioxide.It is also an important biological messenger and transmitter.
Chemical Properties
Nitric oxide is a colorless gas with a sharp, sweet odor; brown at high concentration in air. Shipped as a nonliquefied compressed gas.
Chemical Properties
Nitric oxide,NO, also known as nitrogen oxide and nitrogen monoxide, is a colorless gas that will react with oxygen at room temperature to form nitrogen dioxide, N202, a reddish-brown gas.It is soluble in water and alcohol and is used primarily to form other compounds.
Physical properties
Colorless gas; paramagnetic; density 1.3402 g/L; slightly heavier than air, air density 1.04 (air=1); liquefies at -151.8°C to a blue liquid; the refractive index of the liquid 1.330 at -90°C; the density of the liquid 1.269 g/mL at -150.2°C; solidifies at -163.6°C to a bluish-white snow-like solid; critical temperature -94°C; critical pressure 65 atm; slightly soluble in water, 4.6 mL gas dissolves in 100 mL water at 20°C while 7.34 mL and 2.37 mL dissolve in the same volume of water at 0 and 60°C, respectively; more soluble in alcohol than water; soluble in carbon disulfide, and in ferrous sulfate solution (reacts).
History
Nitric oxide was prepared in 1772 by Joseph Priestley (1733–1804) and described in his volumes Experiments and Observations of Different Kinds of Air published between 1774 and 1786. Priestley called nitric oxide nitrous air, nitrogen dioxide nitrous acid vapor, and nitrous oxide phlogisticated nitrous air, but also referred to the latter as diminished nitrous air. He observed the change of clear nitric oxide to red nitrogen dioxide. Priestley prepared nitric oxide by reacting nitric acid with a metal such as copper: 3Cu(s) + 8HNO3(aq) → 2NO(g) + 3Cu(NO3)2(aq) + 4H2O(l).
Uses
Uses
Nitric oxide is produced as an intermediateusing the Ostwald method to make nitric acid (see Nitric Acid). Nitrogen compoundsproduced from nitric acid are use to manufacture fertilizers, explosives, and other chemicals.
Uses
Nitric oxide is used as an intermediate in themanufacture of nitric acid, in the preparationof metal nitrosyls, in bleaching of rayon,and in incandescent lamps. It is produced byheating air at high temperatures.
Production Methods
Nitric oxide is commercially produced by the catalytic oxidation of ammonia using aplatinum catalyst: 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g).
Definition
A colorless gas that is insoluble in water but dissolves in a solution containing iron(II) ions owing to the formation of the complex ion (FeNO)2+: the nitrogen monoxide can be released by heating. Nitrogen monoxide is prepared by the action of nitric acid on copper turnings; the impure product can be purified by using a solution of iron(II) ions to absorb the product. Commercially, nitrogen monoxide is prepared by the catalytic oxidation of ammonia or by the direct union of nitrogen and oxygen in an electric arc. Nitrogen monoxide is the most heat-stable of the oxides of nitrogen, only decomposing above 1000°C. At ordinary temperatures it combines immediately with oxygen to give nitrogen dioxide:2NO(g) + O2(g) → 2NO2(g).
Definition
ChEBI: Nitric Oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom.
Biological Functions
Nitric oxide is a small, unstable free radical that acts as a
biological messenger in many physiological responses.
Because it can diffuse freely in all directions from its site
of origin, regulation of the activity of nitric oxide is primarily
through control of its synthesis. Formation of nitric
oxide occurs through oxidation of the amino acid Larginine,
a reaction catalyzed by the enzyme nitric oxide
synthase (NOS), to produce nitric oxide and L-citrulline.
The forms of NOS differ in their cellular location and
expression (constitutive expression versus inducible expression).
Activation of synthesis of the inducible form of NOS results in continued synthesis of nitric oxide for
several hours. Inhibitors of NOS are analogues of arginine,
including L-Nw nitroarginine (L- NNA) and L-Nw
methylarginine (L-NMA), both of which decrease nitric
oxide synthesis.
Physiological sites proposed for nitric oxide action
include the immune system, where nitric oxide acts as a
cytostatic agent, is tumoricidal, and can inhibit viral
replication. In the cardiovascular system, nitric oxide is
the biological mediator of vasodilator responses to
agents such as acetylcholine and bradykinin, which act
as receptors on endothelial cells to activate NOS and
stimulate nitric oxide production. Diffusible nitric oxide
then activates guanylate cyclase in vascular smooth
muscle cells, leading to the production of cyclic guanosine
monophosphate (GMP) and vasodilation. In the
brain, stimulation of N-methyl-D-aspartate receptors on neurons leads to activation of the brain form of NOS
and stimulates production of nitric oxide. The function
of brain nitric oxide is thought to involve actions as
a retrograde neurotransmitter whereby nitric oxide
diffuses back to the presynaptic neuron to activate
guanylate cyclase and increase cyclic GMP levels.
Through these retrograde actions nitric oxide is thought
to play a role in the neural circuitry involved in memory.
Even though nitric oxide is the physiological mediator
of a variety of responses, excess nitric oxide is toxic to
many cells as a result of its role in the production of peroxynitrite
and resultant lipid oxidation. Inhibitors of the
NOS enzyme are in clinical trials for the treatment of hypotension
associated with septic shock. Administration
of low concentrations of nitric oxide through respiratory
ventilators has been implemented to treat persistent pulmonary
hypertension of the newborn.
General Description
A colorless gas. Noncombustible but accelerates the burning of combustible material. Vapors heavier than air. Very toxic by inhalation and skin absorption. Heating the containers may cause them to rupture violently and rocket.
Nitric oxide was discovered by Van Helmont in 1620. It occurs in the exhaust gases from automobiles along with other oxides of nitrogen, at trace concentrations. It also is found in minute quantities in the upper atmosphere, resulting from the oxidation of nitrogen in the presence of ionizing radiation or by electric discharge. Nitric oxide is the most stable oxide of nitrogen. It is used as an intermediate or as a starting reactant in the production of many nitrogen compounds, including nitrogen dioxide, nitric acid and nitrosyl chloride.
Air & Water Reactions
Combines very rapidly with oxygen in the air to form nitrogen dioxide. Nitrogen dioxide reacts with water to form nitric acid and NITRIC OXIDE, reacts with alkalis to form nitrates and nitrites [Merck 11th ed. 1989].
Reactivity Profile
NITRIC OXIDE can serve as both an oxidizing agent and as a reducing agent. Sustains the combustion of powdered aluminum [Mellor 5:209-212. 1946-47]. Enflames or explodes when mixed with vapors of carbon disulfide [Mellor 8, Supp. 2:232. 1967]. Reacts vigorously with sodium monoxide above 100°C [Mellor 2, Supp. 2:629. 1961]. Reacts on contact with oxygen at room temperature to form brown gaseous nitrogen dioxide. Reacts with alkalis to form nitrates and nitrites [Merck 11th ed. 1989]. The liquid is very sensitive to detonation in the presence of water.
Hazard
Supports combustion. Toxic by inhalation, strong irritant to skin and mucous membranes. Hypoxia/cyanosis, nitrosyl-hemoglobin formation, and upper respiratory tract irritant.
Health Hazard
Can cause death or permanent injury after a very short exposure to small quantities. Irritant of eyes, nose, throat; can cause unconsciousness. NITRIC OXIDE forms acids in the respiratory system which are irritating and cause congestion in the lungs. Concentrations of 60-150 ppm cause immediate irritation of the nose and throat with coughing and burning in the throat and chest. 6-24 hours after exposure, labored breathing and unconsciousness may result. Concentrations of 100-150 ppm are dangerous for short exposure of 30-60 minutes. Concentrations of 200-700 ppm may be fatal after very short exposure.
Health Hazard
Nitric oxide is an irritant to the eyes, nose,and throat. Inhalation of this gas causesmethemoglobinemia. Its actions are somewhat similar to those of carbon monoxide.It binds with hemoglobin (Hb) in bloodto form metheglobin (NOHb), affecting thetransportation of oxygen to body tissues andorgans:Animal experiments indicate nitric oxide tobe much less toxic than nitrogen dioxide.However, because of its spontaneous oxidation to highly toxic nitrogen dioxide, nitricoxide should be viewed as a severe healthhazard.
Fire Hazard
Noncombustible gas; burns with fuels, hydrocarbons, or when heated with hydrogen. Nitric oxide reacts violently with carbon disulfide vapors, producing green luminous flame; with fluorine, it produces a pale yel low flame. It explodes when mixed with ozone, chlorine monoxide, or a nitrogen tri halide. Reactions with many pyrophoric met als produce incandescence. Reaction with amorphous boron produces brilliant flashes.
Materials Uses
Nitric oxide is noncorrosive, and most common structural materials may be used. However, in the presence of moisture and oxygen, corrosive conditions will develop as a result of the formation of nitric and nitrous acids. Prior to use, systems to contain nitric oxide must first be purged with an inert gas. Where air contamination cannot be eliminated, stainless steel should be used.
Safety Profile
A poison gas. A severe eye, skin, and mucous membrane irritant. A systemic irritant by inhalation. Mutation data reported. Exposure may occur whenever nitric acid acts upon organic material, such as wood, sawdust, and refuse; it occurs when nitric acid is heated, and when organic nitro compounds are burned, for example, celluloid, cellulose nitrate (guncotton), and dynamite. The action of nitric acid upon metals, as in metal etchng and pickling, also liberates the fumes. In hgh-temperature weldmg, as with the oxyacetylene or electric torch, the nitrogen and oxygen of the air unite to form oxides of nitrogen. Automobile exhaust and power plant emissions are also sources of NOx. Exposure occurs in many manufacturing nitric and nitrous acids. This is the action that takes place deep in the respiratory system. The acids formed are irritating and can cause congestion in the throat and bronchi and edema of the lungs. The acids are neutralized by the alkalies present in the tissues, with the formation of nitrates and nitrites. The latter may cause some arterial ddation, fall in blood pressure, headache, and dizziness, and there may be some formation of methemoglobin. However, the nitrite effect is of secondary importance. Because of their relatively low solubllity in water, the nitrogen oxides are initially only slightly irritating to the mucous membranes of the upper respiratory tract. Their warning power is therefore low, and dangerous amounts of the fumes may be breathed before the worker notices any real discomfort. Higher concentrations (60-150 ppm) cause immediate irritation of the nose and throat, with coughing and burning in the throat and chest. These symptoms often clear upon breathing fresh air, and the worker may feel well for several hours. Some 6-24 hours after exposure, a sensation of tightness and burning in the chest develops, followed by shortness of breath, sleeplessness, and restlessness. Dyspnea and air hunger may increase rapidly with development of cyanosis and loss of consciousness followed by death. In cases that recover from the pulmonary edema, there is usually no permanent disabiltty, but pneumonia may develop later. Concentrations of 100-150 ppm are dangerous for short exposures of 30-60 minutes. Concentrations of 200-700 ppm may be fatal after even very short exposures. Continued exposure to low concentrations of the fumes, insufficient to cause pulmonary edema, is said to result in chronic irritation of the respiratory tract, with cough, headache, loss of appetite, dyspepsia, corrosion of the teeth, and gradual loss of strength. Exposure to NOx is always potentially serious, and persons so exposed should be hours. An oxidizer. The liquid is a sensitive explosive. Explosive reaction with carbon disulfide (when ignited), methanol (when ignited), pentacarbonyl iron (at 50℃), phosphine + oxygen, sodium diphenylketyl, dichlorine oxide, fluorine, nitrogen trichloride, ozone, perchloryl fluoride (at 100-300°C), vinyl chloride. Reacts to form explosive products with dienes (e.g., 1,3- butadiene, cyclopentadiene, propadiene). Can react violently with acetic anhydride, Al, amorphous boron, BaO, BCl3, CsHC2, calcium, carbon + potassium hydrogen tartrate, charcoal, Cl0, pyrophoric chromium, 1,2-dichloroethane, dichloroethylene, ethylene, fuels, hydrocarbons, hydrogen + oxygen, NasO, uns-dimethyl hydrazine, NH3, CHCl3, Fe, Mg, Mn, CH2Cl2, olefins, phosphorus, PNH2, PH3, potassium, potassium sulfide, propylene, rubidum acetylide, Na, S, tungsten carbide, trichloroethylene, 1,1,1- trichloroethane, uns-tetrachloroethane, uranium, uranium dicarbide. Wdl react with water or steam to produce heat and corrosive fumes; can react vigorously with reducing materials. processes when nitric acid is made or used. Oxides of nitrogen have been implicated as a cause of acid rain. The oxides of nitrogen are somewhat soluble in water, reacting with it to form
Potential Exposure
Nitric oxide is used in the manufacture of nitric acid; it is also used in the bleaching of rayon; it is a raw material for nitrosyl halide preparation.
Physiological effects
Nitric oxide with the attendant formation of
nitrogen dioxide results in a strong respiratory
irritant that may be fatal. Symptoms may be
moderate at first and include tightness in the
chest, headaches, irritation of the eyes, nausea,
and a slow loss of strength. Delayed symptoms
may be severe and cause increased difficulty in
breathing, chemical pneumonitis, and pulmonary
edema. Untreated cases could lead to
eventual death. Exposure to 100 ppm to 150
ppm of nitrogen oxides for 30-60 minutes could
lead to delayed pulmonary edema, and a few
breaths of nitrogen oxides in 200 ppm to 700
ppm concentrations may result in fatal pulmonary
edema after 5-8 hours have passed.
Acute exposure to nitric oxide alone at high
levels results in the rapid formation of methemog[
obin. Nitric oxide itself does not have the
marked irritant effect of nitrogen dioxide. However,
due to the rapid formation of methemoglobin,
greater lethality can be associated with
high acute levels of exposure to nitric oxide
than with nitrogen dioxide exposures. Initial
symptoms such as muscular tremors, drowsiness,
a brownish-blue hue to the mucous membranes,
increased heart and respiratory rate,
vertigo, and vomiting can occur at methemoglobin
levels of 30 percent to 40 percent. Coma
and death can ensue when methemoglobin levels
reach 70 percent to 90 percent.
ACGIH recommends the Threshold Limit
Value-Time-Weighted Average (TLV-TWA)
of 25 ppm (31 mg/m3
) for nitric oxide. The
TLV-TWA is the time-weighted average concentration
for a normal 8-hour workday and a
40-hour workweek, to which nearly all workers
may be repeatedly exposed, day after day, without
adverse effect.
OSHA lists the 8-hour Time-Weighted Average-
Permissiable Exposure Limit (TWA-PEL)
of 25 ppm (30 mg/m3
) for nitric oxide. TWAPEL
is the exposure limit that shall not be exceeded
by the 8-hour TWA in any 8-hour work
shift of a 40-hour workweek. At normal ambient
temperatures, nitric oxide combines with
atmospheric oxygen to form nitrogen dioxide at
a rate dependent on the concentration of oxygen
and the square of the concentration of nitric
oxide.
Environmental Fate
Nitric oxide is converted spontaneously in the air to nitrogen dioxide; hence, some of the latter gas is present whenever nitric oxide is found in air (at concentrations below 50 ppm). Nitric oxide is a contributor to photochemical air pollution.
storage
Nitric oxide should only be used in well-ventilated areas. Valve protection caps and valve outlet threaded plugs must remain in place unless the container is secured and the valve outlet piped to the point of use. Do not drag, slide, or roll cylinders. Use a suitable hand truck to move cylinders. Use a pressure reducing regulator when connecting a cylinder to lower pressure (1000 psig or 6900 kPa) piping systems. Do not heat a cylinder of nitric oxide by any means to increase the discharge rate from the cylinder. Use a check valve or trap in the discharge line to prevent hazardous reverse flow into the cylinder.
Shipping
UN1660/124 Nitric oxide, compressed, Hazard Class: 2.3; Labels: 2.3-Poisonous gas, 5.1-Oxidizer, 8-Corrosive material, Inhalation Hazard Zone A. Cylinders must be transported in a secure upright position, in a wellventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.
Purification Methods
Bubble the gas through 10M NaOH which removes NO2. It can also be freed from NO2 by passage through a column of Ascarite followed by a column of silica gel held at -197oK. The gas is dried with solid NaOH pellets or by passing through silica gel cooled at -78o, followed by fractional distillation from a liquid N2 trap. This purification does not eliminate nitrous oxide. Other gas scrubbers sometimes used include one containing conc H2SO4 and another containing mercury. It is freed from traces of N2 by the freeze and thaw method. [Blanchard Inorg Synth II 126 1946, Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 485-487 1963.] TOXIC.
Toxicity evaluation
A cytotoxic free radical, nitric oxide impairs mitochondrial ATP synthesis by inhibiting the citrate cycle and other cellular mechanisms of electron transport.
Incompatibilities
A strong oxidizer but may also act as a reducing agent. Explosive reaction with nitrogen trichloride, ozone, carbon disulfide; pentacarbonyl iron; chlorine monoxide. Incompatible with halogens, combustibles, metals, oil, alcohols, chlorinated hydrocarbons (e.g., trichloroethylene), reducing agents (such as NH3), oxygen, fluorine, metals. Reacts with water to form nitric acid. Rapidly converted in air to nitrogen dioxide. Combines very rapidly with oxygen in the air to form nitrogen dioxide. Nitrogen dioxide reacts with water to form nitric acid and nitric oxide, reacts with alkalis to form nitrates and nitrites.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Incineration with added hydrocarbon fuel, controlled so as to produce elemental nitrogen, CO2, and water. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/ mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
References
1.https://en.wikipedia.org/wiki/Nitric_oxide 2.https://www.drugs.com/mtm/nitric-oxide-inhalation-gas.html 3.https://www.britannica.com/science/nitric-oxide 4.http://pediatrics.aappublications.org/content/106/2/344 5.http://www.mensfitness.com/nutrition/supplements/supplement-guide-nitric-oxide 6.https://www.chemicalbook.com/productchemicalpropertiescb5433122.htm
GRADES AVAILABLE
Nitric oxide is available in a minimum purity of 98.5 percent or 99.0 percent.
NITRIC OXIDE Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 7724 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32343 | 55 |
| Central China Special Gas (CCSG) Co., Ltd | 0734-8755555 15674722888 | lyq@ccsg.cn | China | 282 | 58 |
| Guangzhou Yuejia Gas Co., Ltd | 400-6377517 19876107228 | linfeng@yigas.cn | China | 39 | 58 |
| Shangchem Co., Ltd. | +86-21-68182121 | China | 882 | 52 | |
| skychemical Co.Ltd | 021-20958197 20965099 | sales@skychemical.com | China | 1183 | 57 |
| Beijing HuaMeiHuLiBiological Chemical | 010-56205725 | waley188@sohu.com | China | 12335 | 58 |
| Shanghai T&W Pharmaceutical Co., Ltd. | +86 21 61551611 | China | 9891 | 58 |
Related articles
- Is Nitric oxide (NO) polar or non-polar?
- Nitric oxide (NO) is a weakly polar molecule. NO is a polar molecule with a dipole moment equal to 0.15 D.
- Dec 21,2023
- Synthesis and Biological functions of NO
- NO is a 30Da free radical that is a colorless gas with a melting point of -163.6°C. NO has one unpaired electron and is parama....
- Jun 24,2022
10102-43-9(NITRIC OXIDE)Related Search:
1of4