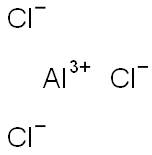
Aluminum chloride
- CAS No.
- 7446-70-0
- Chemical Name:
- Aluminum chloride
- Synonyms
- AlCl3;ALUMINIUM CHLORIDE;ALUMINIUM TRICHLORIDE;ALUMINUM TRICHLORIDE;Aluminiumchlorid;ALUMINIUM CLORIDE;Aluminum chloride, anhydrous;Aluminium chloride, anhydrous, powder, extra pure, 98.5%;Pearsall;NSC 143016
- CBNumber:
- CB6247076
- Molecular Formula:
- AlCl3
- Molecular Weight:
- 133.34
- MDL Number:
- MFCD00003422
- MOL File:
- 7446-70-0.mol
- MSDS File:
- SDS
| Melting point | 194 °C | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boiling point | 180°C | ||||||||||||||
| Density | 2.44 | ||||||||||||||
| vapor pressure | 1 mm Hg ( 100 °C) | ||||||||||||||
| Flash point | 88 °C | ||||||||||||||
| storage temp. | Store below +30°C. | ||||||||||||||
| solubility | H2O: soluble | ||||||||||||||
| form | powder | ||||||||||||||
| Specific Gravity | 2.44 | ||||||||||||||
| color | Yellow to gray | ||||||||||||||
| PH Range | 2.4 | ||||||||||||||
| PH | 2.4 (100g/l, H2O, 20℃) | ||||||||||||||
| Odor | Hydrogen chloride odor detectable when exposed to moist air | ||||||||||||||
| Water Solubility | reacts | ||||||||||||||
| Sensitive | Moisture Sensitive | ||||||||||||||
| Crystal Structure | AlCl3 type | ||||||||||||||
| crystal system | Monoclinic | ||||||||||||||
| Merck | 14,337 | ||||||||||||||
| Space group | C2/m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits | NIOSH: TWA 2 mg/m3 | ||||||||||||||
| Stability | Stable, but reacts violently with water. Prolonged storage may lead to pressure build-up - vent container periodically. Incompatible with alcohols and a variety of other materials (see complete MSDS sheet for full list). | ||||||||||||||
| InChIKey | VSCWAEJMTAWNJL-UHFFFAOYSA-K | ||||||||||||||
| CAS DataBase Reference | 7446-70-0(CAS DataBase Reference) | ||||||||||||||
| EWG's Food Scores | 4-6 | ||||||||||||||
| FDA UNII | LIF1N9568Y | ||||||||||||||
| ATC code | D10AX01 | ||||||||||||||
| NIST Chemistry Reference | Aluminum trichloride(7446-70-0) | ||||||||||||||
| EPA Substance Registry System | Aluminum chloride (7446-70-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314 | |||||||||
| Precautionary statements | P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338-P363 | |||||||||
| Hazard Codes | C,Xi,T | |||||||||
| Risk Statements | 36/38-34-62-51/53-48/23/24-40-23/24/25-14-48/25-48/20-52-36/37/38-52/53-48/23/24/25-60 | |||||||||
| Safety Statements | 26-45-28-7/8-36/37/39-61-23-28A-53 | |||||||||
| RIDADR | UN 3264 8/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | BD0525000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28273200 | |||||||||
| Toxicity | LD50 oral (rat) 3730 mg/kg LD50 skin (rabbit) >2 g/kg TLV-TWA (ACGIH) 2 mg(Al)/m3 |
|||||||||
| NFPA 704 |
|
Aluminum chloride price More Price(78)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.01081 | Aluminium chloride anhydrous powder sublimed for synthesis | 7446-70-0 | 100g | $58.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.01081 | Aluminium chloride anhydrous powder sublimed for synthesis | 7446-70-0 | 500g | $84 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.01081 | Aluminium chloride anhydrous powder sublimed for synthesis | 7446-70-0 | 1kg | $97.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.01081 | Aluminium chloride anhydrous powder sublimed for synthesis | 7446-70-0 | 50kg | $729 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.45019 | Aluminium chloride (anhydrous, powder, ground) Msynth?plus | 7446-70-0 | 1kg | $67.2 | 2022-05-15 | Buy |
Aluminum chloride Chemical Properties,Uses,Production
Description
White or light-yellow crystalline solid (or amorphous solid depending on the method of production); odor of HCl; hygroscopic; melts at 190°C at 2.5 atm; sublimes at 181.2°C; density 2.44 g/cm3 at 25°C; decomposes in water evolving heat; soluble in HCl; soluble in many organic solvents, including absolute ethanol, chloroform, carbon tetrachloride and ether; slightly soluble in benzene.

Aluminum chloride is important industrial chemical. Anhydrous Aluminum chloride is used as the catalyst in variety of Friedel-Crafts type of reactions. Large amounts of ethylbenzene are prepared in this way and are used to make styrene.
C6H6 + CH3CH2Cl + AlCl3→C6H5CH2CH3 + H+ + [AlCl4]-
Aluminum chloride is also used in the manufacture of anthraquinone (used in dyestuffs industry) and dodecylbenzene (used to make detergents) and in the isomerisation of hydrocarbons (petroleum industry).
Aluminum chloride reacts vigorously with water and fumes in air. It is used as a catalyst in cracking petroleum and in organic synthesis.
Uses
Aluminum chloride has extensive commercial applications. It is used primarily in the electrolytic production of aluminum. Another major use involves its catalytic applications in many organic reactions, including Friedel-Crafts alkylation, polymerization, isomerization, hydrocracking, oxidation, decarboxylation, and dehydrogenation. It is also used in the production of rare earth chlorides, electroplating of aluminum and in many metal finishing and metallurgical operations.
Preparation
Aluminum chloride is made by chlorination of molten aluminum at temperatures between 650 to 750°C;
2 Al + 3Cl2→ 2AlCl3
or by chlorination of alumina (bauxite or clay) at 800°C in the presence of a reducing agent, such as carbon or CO. It can be prepared by similar high temperature chlorination of bauxite in the presence of a chlorinated organic reductant such as CCl4.
A pelletized mixture of clay, lignite and a small amount of NaCl is chlorinated at 900°C, producing gaseous AlCl3 (Toth process). Alternatively, alumina is mixed with about 20% by weight carbon and a small amount of sodium salt. The mixture is chlorinated at 600°C (Bayer process).
In the laboratory, anhydrous AlCl3 can be prepared by heating the metal with dry HCl gas at 150°C. The product sublimes and deposits in the cool air condenser. Unreacted HCl is vented out.
Reaction
Reacts with calcium and magnesium hydrides in tetrahydrofuran forming tetrahydro aluminates, Ca(AlH4)2; reacts with hydrides of alkali metals in ether forming aluminum hydride;
Hydrolyzes in chilled, dilute HCl forming aluminum chloride hexahydrate, AlCl3⋅6H2O; reacts violently with water, evolving HCl,
AlCl3 + H2O ——› Al(OH)3 + HCl ↑
Chemical Properties
Aluminum chloride is a noncombustible but highly reactive whitish-gray, yellow, or green powder or liquid. Strong, acidic, irritating odor like hydrochloric acid.The vapor consists of double molecules Al2Cl6 . Soluble in water.
Uses
Astringent (topical).
Uses
Aluminum chloride is used as a catalyst in many organic reactions.
Uses
A yellowish-white crystalline or granular powder made by passing chlorine gas over alumina in a heated state and collecting the product by sublimation. Aluminum chloride was occasionally used in gold and platinum toning baths.
Definition
aluminium chloride: A whitishsolid, AlCl3, which fumes in moist airand reacts violently with water (togive hydrogen chloride). It is knownas the anhydrous salt (hexagonal; r.d.2.44 (fused solid); m.p. 190°C (2.5atm.); sublimes at 178°C) or the hexahydrateAlCl3.6H2O (rhombic; r.d.2.398; loses water at 100°C), both ofwhich are deliquescent. Aluminiumchloride may be prepared by passinghydrogen chloride or chlorine overhot aluminium or (industrially) bypassing chlorine over heated aluminiumoxide and carbon. The chlorideion is polarized by the smallpositive aluminium ion and thebonding in the solid is intermediatebetween covalent and ionic. In theliquid and vapour phases dimer moleculesexist, Al2Cl6, in which thereare chlorine bridges making coordinatebonds to aluminium atoms (seeformula). The AlCl3 molecule can alsoform compounds with other moleculesthat donate pairs of electrons(e.g. amines or hydrogen sulphide);i.e. it acts as a Lewis acid. At hightemperatures the Al2Cl6 molecules inthe vapour dissociate to (planar)AlCl3 molecules. Aluminium chlorideis used commercially as a catalyst inthe cracking of oils. It is also a catalystin certain other organic reactions,especially the Friedel–Craftsreaction.
General Description
Aluminum chloride may be manufactured by chlorination of liquid aluminum in ceramic lined reaction vessels at 600-700oC.
Reactivity Profile
ALUMINUM CHLORIDE behaves as an acidic salt. Self-reactive. After long storage in closed containers, explosions often occur upon opening [Chem. Abst. 41:6723d 1947]. Can cause ethylene(also other alkenes) to polymerize violently [J. Inst. Pet. 33:254 1947]. Causes ethylene oxide to rearrange and polymerize, liberating heat [J. Soc. Chem. Ind. 68:179 1949]. Can catalyze violent polymerization of allyl chloride [Ventrone 1971]. Addition to nitrobenzene containing about 5% phenol caused a violent explosion [Chem. Eng. News 31:4915 1953]. Mixtures with nitromethane may explode when organic matter is present [Chem. Eng. News 26:2257 1948].
Hazard
Powerful irritant to tissue; moderately toxic by ingestion. Reacts violently with water, evolving hydrogen chloride gas.
Health Hazard
Contact with the skin or eyes in the presence of moisture causes thermal and acid burns.
Fire Hazard
Behavior in Fire: Reacts violently with water used in extinguishing adjacent fires
Flammability and Explosibility
Aluminum chloride is not flammable but reacts violently with water, so fires involving this substance should be extinguished with carbon dioxide or dry chemicals. Toxic fumes (HCl and reaction products) can be released during fires.
Industrial uses
Aluminum chloride (AlCl3) act as Lewis acids to a wide range of electron-pair donors, and this has led to their widespread use as catalysts. In the important Friedel–Crafts acylation, AlCl3 is used as a strong Lewis acid catalyst in order to achieve the acylation of an aromatic ring.
Industrial uses
Aluminum chloride (AlCl3) is a volatile solid which sublimes at 458 K. The vapour formed on sublimation consists of an equilibrium mixture of monomers (AlCl3) and dimers (Al2Cl6). It is used to prepare the powerful and versatile reducing agent lithium tetrahydridoaluminate (LiAlH4). Aluminium trichloride (AlCl3) act as Lewis acids to a wide range of electron-pair donors, and this has led to their widespread use as catalysts. In the important Friedel-Crafts acylation, AlCl3 is used as a strong Lewis acid catalyst in order to achieve the acylation of an aromatic ring.
Safety Profile
Moderately toxic by ingestion. Experimental teratogenic and reproductive effects. Mutation data reported. The dust is an irritant by ingestion, inhalation, and skin contact. Highly exothermic polymerization reactions with alkenes. Incompatible with nitrobenaenes or nitrobenzene + phenol. Highly exothermic reaction with water or steam produces toxic fumes of HCl. See also ALUMINUM COMPOUNDS, CHLORIDES, and HYDROCHLORIC ACID.
Potential Exposure
It is used as ethylbenzene catalyst, dyestuff intermediate, and detergent alkylate; in making other chemicals and dyes, astringents, deodorants, in the petroleum refining, and the rubber industries
storage
work with this substance should be conducted in a fume hood, and impermeable gloves should be worn at all times when handling AlCl3. Aluminum chloride should be stored in sealed containers under an inert atmosphere in a cool, dry place. Care should be taken in opening containers of this compound because of the possibility of the buildup of HCl vapor from hydrolysis with traces of moisture.
Shipping
UN1726 Aluminum chloride, anhydrous, Hazard class: 8; Labels: 8-Corrosive material. UN2581 Aluminum chloride solution, Hazard class: 8; Labels: 8-Corrosive material
Purification Methods
Sublime it several times in an all-glass system under nitrogen at 30-50mm pressure. It has also been sublimed in a stream of dry HCl and has been subjected to a preliminary sublimation through a section of granular aluminium metal [for manipulative details see Jensen J Am Chem Soc 79 1226 1957]. It fumes in moist air.
Incompatibilities
A strong reducing agent. Contact with air or water forms hydrochloric acid and hydrogen chloride gas. Reaction with water may be violent. Water, alcohol, and alkenes can cause polymerization. Incompatible with nitrobenzene, organic material, and bases. Attacks metal in presence of moisture, forming flammable hydrogen gas.
Waste Disposal
May be sprayed with aqueous ammonia in the presence of ice and, when reaction is complete, flushed down drain with running water.
Aluminum chloride Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | China | 52849 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 299 | 55 |
| Qingdao Trust Agri Chemical Co.,Ltd | +8613573296305 | aroma@qdtrustagri.com | China | 301 | 58 |
| Auschemicals Pty Ltd | +61406202619 | info@auschemicals.com | Australia | 426 | 58 |
| Zhejiang Fengqing Biotechnology Co. , Ltd. | +86-13157026678 +86-13157026678 | MuMu@zjfq.top | China | 52 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57505 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12839 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
Related articles
- Is AlCl3 ionic or covalent?
- AlCl3 is covalent. Aluminum chloride (AlCl3) is a compound that exhibits characteristics of both ionic and covalent bonding, m....
- Mar 19,2024
- Why is AlCl3 a Nonpolar molecule?
- AlCl3 is a nonpolar molecule because each Al-Cl bond is directed at an angle of 120° to each other in a plane; hence, cancelin....
- Jan 4,2024
- Aluminum Chloride: Uses and Solubility
- Aluminum chloride is used widely in different fields and freely soluble in numerous organic.
- Nov 16,2022
Related Qustion
- Q:Is it safe to drink baking soda water?
- A:It’s usually safe to drink. Some people drink baking soda for indigestion and other purposes, but drinking baking soda can be ....
- Mar 19,2024
View Lastest Price from Aluminum chloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-26 | Aluminum chloride
7446-70-0
|
US $2.30 / Kg/Bag | 1KG | 99% | 20tons | qingdao trust agri chemical co.,ltd | |
 |
2024-11-14 | Aluminum chloride
7446-70-0
|
US $1697.00 / T | 1T | 98% | 1-350mt | Baoji Guokang Bio-Technology Co., Ltd. | |
 |
2024-11-07 | Aluminum chloride
7446-70-0
|
US $9.00 / kg | 1kg | 99% | 2000000 | Hebei Jingbo New Material Technology Co., Ltd |
-

- Aluminum chloride
7446-70-0
- US $2.30 / Kg/Bag
- 99%
- qingdao trust agri chemical co.,ltd
-

- Aluminum chloride
7446-70-0
- US $1697.00 / T
- 98%
- Baoji Guokang Bio-Technology Co., Ltd.
-

- Aluminum chloride
7446-70-0
- US $9.00 / kg
- 99%
- Hebei Jingbo New Material Technology Co., Ltd