Description
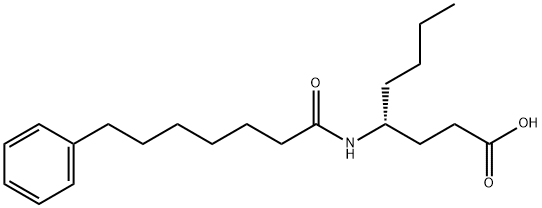
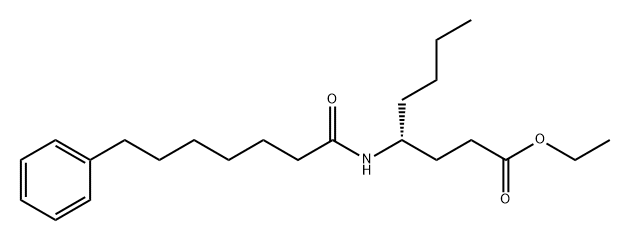
Phospholipase A2 (PLA2) catalyzes the hydrolysis of fatty acids at the sn-2 position of glycerophospholipids, liberating arachidonic acid for subsequent eicosanoid synthesis.1 Three primary types of PLA2 exist: secretory (sPLA2), calcium-dependent cytosolic (cPLA2), and calcium-independent cytosolic (iPLA2).2 Of these three enzymes, cPLA2 is the rate-limiting stimulus for release of arachidonic acid whereas sPLA2 amplifies the action of cPLA2 and regulates phagocytosis and foam cell formation.3 CAY10590, a simple amide based on (R)-γ-norleucine, is a potent and selective inhibitor of sPLA2. It exhibits 95% inhibition (XI(50) = 0.003) of sPLA2 at 0.091 mole fraction without affecting the activities of cPLA2 or iPLA2.4
References
1. Dennis, E.A. Phospholipases The Enzymes XVI,(1983).
2. Dennis, E.A. Diversity of group types, regulation, and function of phospholipase A2 J. Biol. Chem. 269,13057-13060(1994).
3. Balestrieri, B., and Arm, J.P. Group V sPLA2: Classical and novel functions Biochim. Biophys. Acta 1761,1280-1288(2006).
4. Antonopoulou, G., Barbayianni, E., Magrioti, V., et al. Structure-activity relationships of natural and non-natural amino acid-based amide and 2-oxoamide inhibitors of human phospholipase A2 enzymes Bioorg. Med. Chem. 16,10257-10269(2008).