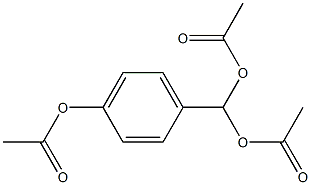
4-Hydroxybenzaldehyde
- CAS No.
- 123-08-0
- Chemical Name:
- 4-Hydroxybenzaldehyde
- Synonyms
- 4-HYDROXYBENZALDEHYDE;PHB;PARA HYDROXY BENZALDEHYDE;Benzaldehyde, 4-hydroxy-;4-Hydroxybenzaldehyde ,99%;FEMA 3984;4-FORMYLPHENOL;p-Formylphenol;p-Oxybenzaldehyde;Hydroxy benzaldehyd
- CBNumber:
- CB6446138
- Molecular Formula:
- C7H6O2
- Molecular Weight:
- 122.12
- MDL Number:
- MFCD00006939
- MOL File:
- 123-08-0.mol
- MSDS File:
- SDS
| Melting point | 112-116 °C (lit.) |
|---|---|
| Boiling point | 191°C 50mm |
| Density | 1,129 g/cm3 |
| vapor pressure | 0.004Pa at 25℃ |
| refractive index | 1.5105 (estimate) |
| FEMA | 3984 | 4-HYDROXYBENZALDEHYDE |
| Flash point | 174°C |
| storage temp. | Store below +30°C. |
| solubility | 13.8g/l |
| form | Crystalline Powder |
| pka | 7.61(at 25℃) |
| color | light yellow to light brown |
| Odor | at 100.00 %. sweet nutty almond balsam woody |
| Odor Type | woody |
| Water Solubility | 13 g/L (30 ºC) |
| Sensitive | Air Sensitive |
| JECFA Number | 956 |
| Merck | 14,4811 |
| BRN | 471352 |
| Stability | Hygroscopic |
| InChIKey | RGHHSNMVTDWUBI-UHFFFAOYSA-N |
| LogP | 1.3 at 23℃ |
| Substances Added to Food (formerly EAFUS) | 4-HYDROXYBENZALDEHYDE |
| CAS DataBase Reference | 123-08-0(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | O1738X3Y38 |
| NIST Chemistry Reference | Benzaldehyde, 4-hydroxy-(123-08-0) |
| EPA Substance Registry System | p-Hydroxybenzaldehyde (123-08-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H318 | |||||||||
| Precautionary statements | P280-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38-R36/37/38 | |||||||||
| Safety Statements | 26-36-24/25-37/39-S24/25 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | CU6475000 | |||||||||
| Autoignition Temperature | 450 °C | |||||||||
| Hazard Note | Irritant/Air Sensitive | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29124900 | |||||||||
| Toxicity | LD50 orally in Rabbit: 2250 mg/kg | |||||||||
| NFPA 704 |
|
4-Hydroxybenzaldehyde price More Price(53)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W398403 | 4-Hydroxybenzaldehyde ≥97%, FG | 123-08-0 | 1kg | $257 | 2024-03-01 | Buy |
| Sigma-Aldrich | W398403 | 4-Hydroxybenzaldehyde ≥97%, FG | 123-08-0 | 5kg | $876 | 2024-03-01 | Buy |
| Sigma-Aldrich | W398403 | 4-Hydroxybenzaldehyde ≥97%, FG | 123-08-0 | 10Kg | $1190 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.04536 | 4-Hydroxybenzaldehyde for synthesis | 123-08-0 | 100g | $69.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.04536 | 4-Hydroxybenzaldehyde for synthesis | 123-08-0 | 250g | $145 | 2024-03-01 | Buy |
4-Hydroxybenzaldehyde Chemical Properties,Uses,Production
description
There are three kinds of isomers of hydroxybenzaldehyde, namely, o-hydroxybenzaldehyde, m-hydroxybenzaldehyde and p-hydroxybenzaldehyde. p-hydroxybenzaldehyde(4-Hydroxybenzaldehyde) is also known as phenol formaldehyde. When precipitated from water, it is white to pale yellow needles. It has aromatic odor. At atmospheric pressure, it can be sublimated without decomposition. The molecular weight is 122.12. Melting point: 115~116 °C. The relative density is 1.129 (130/4 °C). The refractive index is 1.5705 (130 °C). It is slightly soluble in water and benzene, easily soluble in alcohol, ether, acetone, and ethyl acetate with water solubility at 30.5 °C being 1.38 and benzene solubility in 65 °C being 3.68. Intraperitoneal injection of mice: LD50: 500mg/kg.
4-Hydroxybenzaldehyde is an important intermediate of pharmaceutical, perfume, liquid crystal. It can generate anisaldehyde when having reaction with dimethyl sulfate, and can generate hydroxy cinnamic aldehyde upon its reaction with acetaldehyde which can further undergo oxidation to obtain cinnamic acid. The direct oxidation of this product can prepare hydroxybenzoic acid; Its reduction can generate p-hydroxyphenyl rmethanol; both of them can be used as spices; pharmaceutical intermediates; raw material of liquid crystal; other kinds of organic synthesis intermediates with a wide range of applications.
In addition to be used as a spice, m-hydroxybenzaldehyde can also be used as the intermediate of producing other kind of species; it can also be used as a kind of pharmaceutical raw materials for production of phenylephrine hydrochloride, epinephrine, and quinine; nickel plating; chemical analysis reagent (sugar quantitative analysis) ; photographic emulsion and fungicides.
O-hydroxybenzaldehyde, also known as salicylaldehyde, is a kind of colorless oily liquid with special odor and bitter almond flavor. It is chemically active and can have various kinds of reactions such as substitution condensation, oxidation, and Wei Tixi (Wittig) reaction. Its reaction with sulfuric acid generates orange color; its reaction with metal ions is to form a colored chelate. Its reaction with ferric chloride solution yields purple color and it can be reduced to salicyl alcohol. It is mainly used as the raw material for the production of perfume "coumarin" and "Dihydrocoumarins"; it can also be used as the raw material for preparation of violet fragrance; it can be also used as fungicides.
4-Hydroxybenzaldehyde Preparation method: take phenol as the raw material, have chloroform reacted with a phenol sodium salt at 60 °C; or have condensation reaction between phenol and chloral in the presence of potassium carbonate as the catalyst, and then they further undergo decomposition by sodium methylate; it can also be produced by pouring dry hydrogen chloride into the mixture between phenol and hydrogen cyanide in the presence of aluminum chloride as the catalyst and the decomposition reaction in ice water to obtain the finished p-hydroxybenzaldehyde product.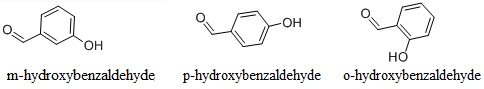
The above information is edited by the Chemicalbook of Dai Xiongfeng.
Chemical Properties
It is colorless crystalline powder. Melting point: 115-116 ℃, the relative density is 1.129 (30/4 ℃). In the air, it is easily to be sublimated. It is easily soluble in alcohol, ether, acetone, and ethyl acetate, slightly soluble in water (at 30.5 ℃, its water solubility is 1.38g/100ml); it can also be dissolved in benzene (at 65 ℃, its solubility in benzene is 3.68g/ml). It has aromatic odor.
synthesis
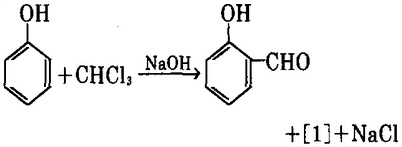
1. use chloroform and phenol as raw materials, have them heated in an alkaline solution for Reimer-Tiemann reaction while generating p-hydroxybenzaldehyde [1] and a small amount of salicylaldehyde (o-hydroxybenzaldehyde).
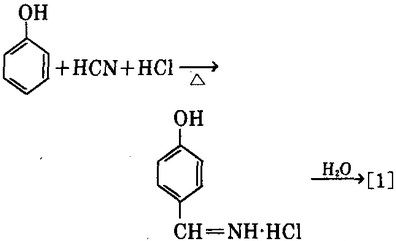
2. use phenol and hydrogen cyanide as the raw materials, in presence of aluminum chloride as the catalyst, have them reacted with anhydrous hydrogen chloride to generate the imine hydrochloric acid of p-hydroxybenzaldehyde, followed by hydrolysis to obtain p-hydroxybenzaldehyde [1]. For the reaction solvent, we can use benzene, chlorobenzene, and paraffins chloro; zinc cyanide can also be used as substitute of hydrogen cyanide.
3. phenol and trichloracetic aldehyde have condensation reaction in the presence of potassium carbonate as the catalyst, and then further undergo decomposition by sodium methylate in dimethyl formamide to obtain the final product.
4. take p-cresol as the starting material, have it reacted with acetic acid to produce para-toluene acetate ester, and then further perform chlorination reaction, hydrolysis reaction to obtain the p-hydroxybenzaldehyde.
5. take nitrotoluene as the raw material, have it reacted with sodium polysulfide with the catalyst to have reduction reaction to produce p-amino benzaldehyde; it further has diazotization reaction with sodium nitrite in sulfuric acid to generate diazonium salt which is then hydrolyzed at 100~110 ℃ to obtain hydroxybenzaldehyde [1].
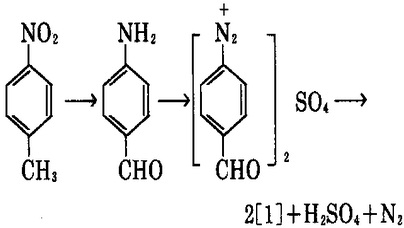
Uses
1. It can be used for the synthesis of organic compounds and medicine.
2. The good is also the intermediate of the pharmaceutical, perfume, and liquid crystal. p-Hydroxybenzaldehyde can be used for the production of TMP (trimethoprim), amoxicillin, cefadroxil cephalosporins, artificial gastrodin, farrerol, bezafibrate, and esmolol; it can also be used for the production of perfume anisaldehyde, vanillin, ethyl vanillin, and raspberry ketone.
3. It is the key raw material for the production of p-hydroxy phenylglycine (p-hydroxy phenylglycine is the key intermediate of the new generation of semi-synthetic penicillin amoxicillin). It is also the key intermediate raw material of the production of pesticides-bromoxynil.
4. p-Hydroxybenzaldehyde(4-Hydroxybenzaldehyde) is an important intermediate of the pharmaceutical industry and the perfume industry.
5. It is an important intermediate of medicine, perfume, and pesticides for the synthesis of drugs like hydroxyl ampicillin, trimethoprim, and 3-methoxy benzaldehyde. It can also used for synthesizing polymers and producing pharmaceutical raw materials.
Chemical Properties
4-Hydroxybenzaldehyde is a yellow to light brown crystalline powder that has a very faint, sweet-woody-balsamic odor and a sweet taste with little or no other flavor impression. The odor is also reported as vanillic/nutty.
Occurrence
It is found as a volatile in several food products, including cherries, grapes, papayas, tomatoes, cheese, beer, rum, brandy, wine, tea and peanuts. Occurs in the form of esters in several plants, notably in wintergreen leaves and the bark of sweet birch.
Uses
4-Hydroxybenzaldehyde maintains bactericidal activity when tested against certain bacteria strains. It also displays antioxidant potential when analyzed through assay. It is widely used starting material for polymers and pharmaceuticals.
Application
p-Hydroxybenzaldehyde is the important intermediates of pharmaceutical industry and spices. In foreign , it's also used for synthesis of bromoxynil and chloroxynil which are kind of herbicides, and also used in the manufacture of bactericide, photographic emulsifier, nickel plating luster agent, liquid crystal, etc; In the pharmaceutical field, it can be used for synthesis of amoxicillin, antibacterial synergistic agent named TMP, 3,4,5-Trimethoxybenzaldehyde,Artificial gastrodia elata, farrerol, esmololhydrochloride; In the spicery field, it can be used for synthesis of spicery,for example: vanillin, ethyl vanillin, piperonal, springaldehyde, p-anisaldehyde, raspberry ketone natural,etc.
Preparation
p-Hydroxybenzaldehyde is prepared by heating sodium phenolate with carbon dioxide under pressure.
Definition
ChEBI: 4-hydroxybenzaldehyde is a hydroxybenzaldehyde that is benzaldehyde substituted with a hydroxy group at position C-4. It has a role as a plant metabolite, a mouse metabolite and an EC 1.14.17.1 (dopamine beta-monooxygenase) inhibitor.
Synthesis Reference(s)
Synthetic Communications, 9, p. 407, 1979 DOI: 10.1080/00397917908064169
General Description
4-hydroxybenzaldehyde occurs naturally in vanilla beans and is one of the keys contributors to the vanilla flavor.
Synthesis
2,3-Dichloro-5,6-dicyano-p-benzoquinone (DDQ 908 mg, 4 mmol) was added to a solution of 4-hydroxybenzyl alcohol (496 mg, 4 mmol) in dioxane (24 mL). The reaction mixture immediately turned deep green (exothermic reaction), and DDQH2 started precipitating within 1 min. Thin layer chromatography (TLC) analysis indicated consumption of starting material after 15 min. The solvent was removed from the yellow reaction mixture in vacuo. Treatment of the residue with CH2Cl2 left DDQH2 undissolved (quantitatively). Filtration followed by evaporation of CH2Cl2 gave 4-hydroxybenzaldehyde (74% yield) which was recrystallized from water. 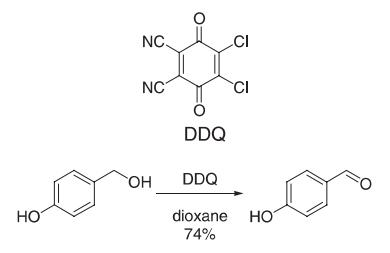
Reference: Becker, H.-D.; Bjork, A.; Alder, E. J. Org. Chem. 1980, 45, 1596?1600.
target
GABA Receptor
Purification Methods
Crystallise it from water (containing some H2SO4). Dry it over P2O5 under vacuum. [Beilstein 8 H 64, 8 IV 251.]
References
[1] HANS DIETER BECKER Erich A Anders Bjoerk. Quinone dehydrogenation. Oxidation of benzylic alcohols with 2,3-dichloro-5,6-dicyanobenzoquinone[J]. The Journal of Organic Chemistry, 1980, 45 9: 1596-1600. DOI:10.1021/jo01297a010.
[2] CHAN WOO KANG. 4-Hydroxybenzaldehyde accelerates acute wound healing through activation of focal adhesion signalling in keratinocytes.[J]. Scientific Reports, 2017: 14192. DOI:10.1038/s41598-017-14368-y.
[3] M. EDDOUKS. Insulin Resistance as a Target of Some Plant-Derived Phytocompounds[J]. Studies in natural products chemistry, 1900, 26 1: 351-373. DOI:10.1016/B978-0-444-63430-6.00011-4.
[4] DOUGLASS F. TABER. Vanillin Synthesis from 4-Hydroxybenzaldehyde[J]. Journal of Chemical Education, 2007, 84 7: 1158. DOI:10.1021/ed084p1158.
[5] MAKOTO KOMIYAMA Hidefumi H. Selective synthesis of 4-hydroxybenzaldehyde with cyclodextrin as catalyst[J]. Macromolecular Rapid Communications, 1981, 2 12: 715-717. DOI:10.1002/marc.1981.030021202.
[6] HIROAKI MATSUDA . Mutagenicity of ozonation and chlorination products from p-hydroxybenzaldehyde[J]. Science of the Total Environment, 1991, 103 2: Pages 141-149. DOI:10.1016/0048-9697(91)90140-A.
4-Hydroxybenzaldehyde Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of5
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Wuwei hailun New Material Technology Co., Ltd. | +86-0935-5322366 +86-13605736198 | hailunchem@vip.163.com | China | 7 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2964 | 58 |
| Ningxia Jinhua Chemical Co.,Ltd | 025-52279164 | info@nxjhchem.com | China | 79 | 58 |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-0519-85551759 +8613506123987 | marketing1@neostarunited.com | China | 8838 | 58 |
| Jiaxing Dongliang Pharmaceutical Technology Co.,Ltd. | +86-0573-84772883 +86-18905731109 | hujidong@hailunchem.com | China | 7 | 58 |
| Mainchem Co., Ltd. | -- | sarah@mainchem.com | China | 6567 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +86-15350851019 +86-15383190639 | admin@86-ss.com | China | 1000 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 | admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 12272 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8811 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
Related articles
- 4-Hydroxybenzaldehyde: A Versatile Compound in Modern Chemistry
- 4-Hydroxybenzaldehyde (4-HBA) is a prominent organic compound widely recognized for its diverse applications in the chemical i....
- Jun 6,2024
- 4-Hydroxybenzaldehyde: Synthesis, applications and bioactivities
- 4-Hydroxybenzaldehyde is one of the three isomers of hydroxybenzaldehyde. It can be found in the orchids Gastrodia elata and G....
- Jun 8,2023
- p-Hydroxybenzaldehyde-Application
- 4-Hydroxybenzaldehyde is one of the three isomers of hydroxybenzaldehyde. It can be found in the orchids Gastrodia elata and G....
- Dec 19,2019
View Lastest Price from 4-Hydroxybenzaldehyde manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-25 | p-Hydroxybenzaldehyde (PHBA)
123-08-0
|
US $0.00 / kg | 1kg | 99% | 500mt | Jinan Finer Chemical Co., Ltd | |
 |
2024-11-25 | 4-Hydroxybenzaldehyde
123-08-0
|
US $100.00-75.00 / kg | 1kg | 99% | 5000Ton | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-11-25 | 4-Hydroxybenzaldehyde
123-08-0
|
US $3.50 / kg | 1kg | ≥99% | 3000tons/month | Hebei Andu Technology Com.,Ltd |
-

- p-Hydroxybenzaldehyde (PHBA)
123-08-0
- US $0.00 / kg
- 99%
- Jinan Finer Chemical Co., Ltd
-

- 4-Hydroxybenzaldehyde
123-08-0
- US $100.00-75.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- 4-Hydroxybenzaldehyde
123-08-0
- US $3.50 / kg
- ≥99%
- Hebei Andu Technology Com.,Ltd
123-08-0(4-Hydroxybenzaldehyde)Related Search:
1of4