Butyl acetate
- CAS No.
- 123-86-4
- Chemical Name:
- Butyl acetate
- Synonyms
- N-BUTYL ACETATE;ACETIC ACID BUTYL ESTER;Acetic acid butyl;Butylacetat;Butyl ethanoate;Butyle;butylacetates;Butile;BUTYLE ACETATE;1-Butyl acetate
- CBNumber:
- CB6671615
- Molecular Formula:
- C6H12O2
Lewis structure
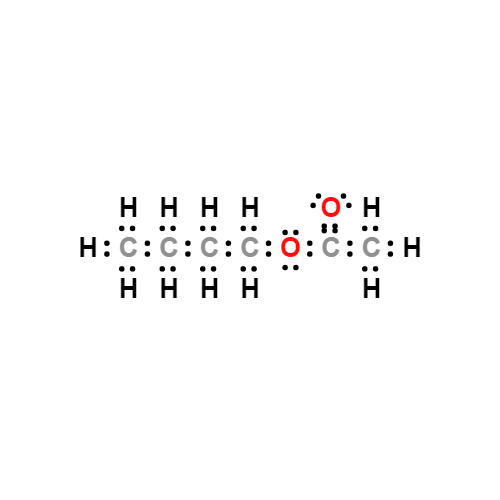
- Molecular Weight:
- 116.16
- MDL Number:
- MFCD00009445
- MOL File:
- 123-86-4.mol
- MSDS File:
- SDS
| Melting point | -78 °C (lit.) |
|---|---|
| Boiling point | 124-126 °C (lit.) |
| Density | 0.88 g/mL at 25 °C (lit.) |
| vapor density | 4 (vs air) |
| vapor pressure | 15 mm Hg ( 25 °C) |
| refractive index |
n |
| FEMA | 2174 | BUTYL ACETATE |
| Flash point | 74 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 5.3g/l |
| form | Liquid |
| Specific Gravity | 0.883 (20/20℃) |
| color | ≤10(APHA) |
| Odor | Characteristic; agreeable fruity (in low concentrations); non residual. |
| PH | 6.2 (5.3g/l, H2O, 20℃)(External MSDS) |
| explosive limit | 1.4-7.5%(V) |
| Odor Threshold | 0.016ppm |
| Odor Type | ethereal |
| Viscosity | 0.83mm2/s |
| Water Solubility | 0.7 g/100 mL (20 ºC) |
| FreezingPoint | -77.9℃ |
| λmax |
λ: 254 nm Amax: 1.0 λ: 260 nm Amax: 0.20 λ: 275 nm Amax: 0.04 λ: 300 nm Amax: 0.02 λ: 320-400 nm Amax: 0.01 |
| Merck | 14,1535 |
| JECFA Number | 127 |
| BRN | 1741921 |
| Henry's Law Constant | 5.79 at 37 °C (static headspace-GC, van Ruth et al., 2001) |
| Dielectric constant | 5.0(20℃) |
| Exposure limits | TLV-TWA 150 ppm (~710 mg/m3) (ACGIH, MSHA, and OSHA); TLV-STEL 200 ppm (~950 mg/m3); IDLH 10,000 ppm (NIOSH). |
| Stability | Stable. Flammable. Incompatible with strong oxidizing agents, strong acids, strong bases. |
| InChIKey | DKPFZGUDAPQIHT-UHFFFAOYSA-N |
| LogP | 1.82-2.3 at 25℃ |
| Substances Added to Food (formerly EAFUS) | BUTYL ACETATE |
| FDA 21 CFR | 172.515; 175.105; 175.320; 177.1200 |
| CAS DataBase Reference | 123-86-4(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 464P5N1905 |
| NIST Chemistry Reference | Acetic acid, butyl ester(123-86-4) |
| EPA Substance Registry System | n-Butyl acetate (123-86-4) |
| Cosmetics Info | Butyl Acetate |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H226-H336 | |||||||||
| Precautionary statements | P210 | |||||||||
| Risk Statements | 10-66-67-R67-R66-R10 | |||||||||
| Safety Statements | 25-S25 | |||||||||
| RIDADR | UN 1123 3/PG 3 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 150 ppm (710 mg/m3), STEL: 200 ppm (950 mg/m3) | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | AF7350000 | |||||||||
| Autoignition Temperature | 790 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2915 33 00 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | LD50 orally in rats: 14.13 g/kg (Smyth) | |||||||||
| IDLA | 1,700 ppm | |||||||||
| NFPA 704 |
|
Butyl acetate price More Price(56)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W217417 | Butyl Acetate natural, ≥98%, FG | 123-86-4 | 100g | $76 | 2024-03-01 | Buy |
| Sigma-Aldrich | W217417 | Butyl Acetate natural, ≥98%, FG | 123-86-4 | 1kg | $128 | 2024-03-01 | Buy |
| Sigma-Aldrich | W217417 | Butyl Acetate natural, ≥98%, FG | 123-86-4 | 4kg | $383 | 2024-03-01 | Buy |
| Sigma-Aldrich | W217409 | Butyl Acetate ≥99%, FCC, FG | 123-86-4 | 1kg | $77.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | W217409 | Butyl Acetate ≥99%, FCC, FG | 123-86-4 | 9kg | $308 | 2024-03-01 | Buy |
Butyl acetate Chemical Properties,Uses,Production
Description
Butyl acetate is a clear, flammable ester of acetic acid that occurs in n-, sec-, and tert- forms (INCHEM, 2005). Butyl acetate isomers have a fruity, banana-like odor (Furia, 1980). Isomers of butyl acetate are found in apples (Nicholas, 1973) and other fruits (Bisesi, 1994), as a well as in a number of food products, such as cheese, coffee, beer, roasted nuts, vinegar (Maarse and Visscher, 1989). Butyl acetate is manufactured via esterification of the respective alcohol with acetic acid or acetic anhydride (Bisesi, 1994). N-butyl acetate is used as a solvent for nitrocellulose-based lacquers, inks, and adhesives. Other uses include manufacture of artificial leathers, photographic film, safety glass, and plastics (Budavari, 1996). Isomers of butyl acetate are also used as flavoring agents, in manicure products, and as larvicides (Bisesi, 1994). The tert-isomer has been used as a gasoline additive (Budavari, 1996). It may be used as a synthetic fruit flavoring in candy, ice cream, cheeses, and baked goods (Dikshith, 2013).
Chemical Properties
Butyl acetate is a colorless or yellowish liquid with a strong fruity odor. burning and then sweet taste reminiscent of pineapple. It occurs in many fruits and is a constituent of apple aromas. Butyl acetate is incompatible with strong oxidizing agents, strong acids, and strong bases.
There are 4 isomers. At 20 °C, the density of the n-butyl isomer is 0.8825 g/ cm3, and the density of the sec-isomer is 0.8758 g/cm3 (Bisesi, 1994). The n-butyl isomer is soluble in most hydrocarbons and acetone, and it is miscible with ethanol, ethyl ether, and chloroform (Haynes, 2010). It dissolves many plastics and resins (NIOSH, 1981).
Physical properties
Clear, colorless liquid with a strong fruity odor resembling bananas. Sweetish taste as low concentrations (<30 μg/L). Experimentally determined detection and recognition odor threshold concentrations were 30 μg/m3 (6.3 ppbv) and 18 μg/m3 (38 ppbv), respectively (Hellman and Small, 1974). Cometto-Mu?iz et al. (2000) reported nasal pungency threshold concentrations ranged from approximately 550 to 3,500 ppm.
Occurrence
Reported present in rum ether, pears, pear brandy, cider, mango, mountain papaya (C. pubescens), soybean, roasted peanuts and honey and other natural products.
Uses
Butyl acetate is one of the more important derivatives of n-butyl alcohol produced commercially, is employed as a solvent in rapid drying paints and coatings. In some instances, butyl acetate, C6H12O2, has replaced ethoxyethyl acetate due to the latter’s reported toxicity and teratogenicity.
Uses
n-Butyl acetate is used in the manufactureof lacquers, plastics, photographic films, andartificial leathers.
Uses
Butyl Acetate is a flavoring agent which is a clear, colorless liquid possessing a fruity and strong odor. it is sparingly soluble in water and miscible in alcohol, ether, and propylene glycol. it is also termed n-butyl acetate.
Definition
ChEBI: Butyl acetate is the acetate ester of butanol. It has a role as a metabolite. It is functionally related to a butan-1-ol.
Production Methods
Butyl alcohol is combined with acetic acid in the presence of a catalyst such as sulfuric acid. After esterification is complete, the solution is distilled to yield butyl acetate .
Preparation
By esterification of n-butyl alcohol with acetic acid.
Aroma threshold values
Detection: 10 to 500 ppb
Synthesis Reference(s)
Journal of the American Chemical Society, 73, p. 5265, 1951 DOI: 10.1021/ja01155a075
The Journal of Organic Chemistry, 39, p. 3728, 1974 DOI: 10.1021/jo00939a026
General Description
A clear colorless liquid with a fruity odor. Flash point 72 - 88°F. Density 7.4 lb / gal (less than water). Hence floats on water. Vapors heavier than air.
Air & Water Reactions
Highly flammable. Very slightly soluble in water.
Reactivity Profile
Butyl acetate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Attacks many plastics. [Handling Chemicals Safely 1980. p. 233].
Hazard
Skin irritant, toxic. Flammable, moderate fire risk. Eye and upper respiratory tract irritant.
Health Hazard
The narcotic effects of n-butyl acetate isgreater than the lower alkyl esters of aceticacid. Also, the toxicities and irritant actionsare somewhat greater than n-propyl, iso propyl, and ethyl acetates. Exposure to its vapors at about 2000 ppm caused mild irritation of the eyes and salivation in test animals. A 4-hour exposure to 14,000 ppm wasfatal to guinea pigs. In humans, inhalation of 300-400 ppm of n-butyl acetate may produce moderate irritation of the eyes and throat, and headache.
Health Hazard
Exposures to n-butyl acetate cause harmful effects that include, but are not limited to, coughing and shortness of breath. High concentrations have a narcotic effect, with symp toms such as sore throat, abdominal pain, nausea, vomiting, and diarrhea. High concen trations of n-butyl acetate cause severe poisoning. Prolonged periods of exposure cause adverse effects to the lungs, the nervous system, and the mucous membranes. Repeated skin contact causes skin dryness or cracking, and dermatitis.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Biochem/physiol Actions
Taste at 30 ppm
Safety Profile
Moderately toxic by intraperitoneal route. Mdly toxic by inhalation and ingestion. An experimental teratogen. A skin and severe eye irritant. Human systemic effects by inhalation: conjunctiva irritation, unspecified nasal and respiratory system effects. A mild allergen. High concentrations are irritating to eyes and respiratory tract and cause narcosis. Evidence of chronic systemic toxicity is inconclusive. Flammable liquid. Moderately explosive when exposed to flame. Ignites on contact with potassium tert-butoxide. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid and irritating fumes. See also ESTERS.
Potential Exposure
n-Butyl acetate is an important solvent in the production of lacquers, leather and airplane dopes, and perfumes. It is used as a solvent and gasoline additive. sec-Butyl acetate is used as a widely used solvent for nitrocellulose, nail enamels and many different purposes. tert-Butyl acetate is common industrial solvent used in the making of lacquers, artificial leather, airplane dope, perfume; and as a food additive. Isobutyl acetate is used as a solvent and in perfumes and artificial flavoring materials
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of saltwater and inducevomiting. Do not make an unconscious person vomit.
Carcinogenicity
There are no indications of mutagenic or cytogenic effects for n-butyl acetate.
Source
Identified as a volatile constituent released by fresh coffee beans (Coffea canephora variety Robusta) at different stages of ripeness (Mathieu et al., 1998). Also identified among 139 volatile compounds identified in cantaloupe (Cucumis melo var. reticulates cv. Sol Real) using an automated rapid headspace solid phase microextraction method (Beaulieu and Grimm, 2001).
storage
n-Butyl acetate should be kept stored in a segregated and approved area. Workers should keep the container in a cool, well-ventilated area, closed tightly, and sealed until ready for use. Workers should avoid all possible sources of ignition/spark at the workplace
Shipping
UN1123 Butyl acetates, Hazard Class: 3; Labels: 3—Flammable liquid.
Purification Methods
Distil, reflux with successive small portions of KMnO4 until the colour persists, dry with anhydrous CaSO4, filter and redistil. [Beilstein 2 IV 143.]
Incompatibilities
All butyl acetates are incompatible with nitrates, strong oxidizers; strong alkalies; strong acids. Butyl acetates may form explosive mixture with air; reacts with water, on standing, to form acetic acid and n-butyl alcohol. Violent reaction with strong oxidizers and potassium-tert-butoxide. Dissolves rubber, many plastics, resins and some coatings. May accumulate static electrical charges, and may cause ignition of its vapors
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Precautions
On exposure to Butyl acetate, immediately wash with plenty of water, also under the eyelids, for at least 15 min. Remove contact lenses. Butyl acetate is flammable in the pres ence of open flames, sparks, oxidizing materials, acids, and alkalis. It poses explosion risk in the presence of mechanical impact. For health safety, management authorities should provide exhaust ventilation facilities at the workplace to keep the airborne concentrations of vapors of Butyl acetate below TLV.
Butyl acetate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of5
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shandong Yanshuo Chemical Co., Ltd. | +86-18678179670 +86-18615116763 | sales@yanshuochem.com | China | 101 | 58 |
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 | sales01@sdlookchemical.com | China | 2737 | 58 |
| PT CHEM GROUP LIMITED | peter68@ptchemgroup.com | China | 35425 | 58 | |
| Yixing Wencheng Chemical Co., Ltd | +86-0510-87501824 +86-13961572207 | David@un-wencheng.com | China | 124 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Hebei Dangtong Import and export Co LTD | +86-13910575315 +86-13910575315 | admin@hbdangtong.com | China | 1000 | 58 |
| AS WATER CO., LTD. | +86-18994963526 +8618994963526 | 1346073549@qq.com | United Kingdom | 176 | 58 |
| Qingdao RENAS Polymer Material Co., Ltd. | +86-0532-86867058 +86-18562606086 | sales@qdrenas.com | China | 476 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
Related articles
- The toxicity of n-Butyl acetate
- n-Butyl Acetate is a clear, colorless liquid with a fruity odor. It is miscible with all conventional solvents such as alcohol....
- Apr 28,2024
- Butyl Acetate: Uses and Commercial Manufacturing Method
- The passage introduces the uses and commercial manufacturing method of Butyl Acetate.
- Nov 18,2022
- Butyl acetate-a Versatile chemical Solvent
- Butyl acetate, also known as butyl ethanoate or n-butyl acetate, is a chemical compound. Butyl acetate is an ester that is a c....
- Nov 18,2019
View Lastest Price from Butyl acetate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-28 | Butyl acetate
123-86-4
|
US $0.00 / KG | 1KG | 99% | 1000mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2024-11-14 | Butyl acetate
123-86-4
|
US $160.00 / KG | 1KG | 98% | 1-20mt | Baoji Guokang Bio-Technology Co., Ltd. | |
 |
2024-10-29 | Butyl acetate
123-86-4
|
US $0.00 / kg | 20kg | 99.0% | 20 tons | Qingdao RENAS Polymer Material Co., Ltd. |
-

- Butyl acetate
123-86-4
- US $0.00 / KG
- 99%
- Jinan Finer Chemical Co., Ltd
-

- Butyl acetate
123-86-4
- US $160.00 / KG
- 98%
- Baoji Guokang Bio-Technology Co., Ltd.
-

- Butyl acetate
123-86-4
- US $0.00 / kg
- 99.0%
- Qingdao RENAS Polymer Material Co., Ltd.
123-86-4(Butyl acetate)Related Search:
1of4








