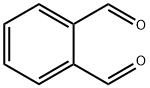
o-Phthalaldehyde
- CAS No.
- 643-79-8
- Chemical Name:
- o-Phthalaldehyde
- Synonyms
- OPA;OPD;PHTHALALDEHYDE;PHTHALDIALDEHYDE;1,2-BENZENEDICARBOXALDEHYDE;O-PHTHALDIALDEHYDE;ORTHO-PHTHALALDEHYDE;1,2-PHTHALIC DICARBOXALDEHYDE;O-PHTHALDEHYDE;Phthaldialdehy
- CBNumber:
- CB6731197
- Molecular Formula:
- C8H6O2
- Molecular Weight:
- 134.13
- MDL Number:
- MFCD00003335
- MOL File:
- 643-79-8.mol
- MSDS File:
- SDS
| Melting point | 55-58 °C(lit.) |
|---|---|
| Boiling point | 83-84 °C (0.7501 mmHg) |
| Density | 1.13 |
| vapor pressure | 0.56Pa at 25℃ |
| refractive index | 1.4500 (estimate) |
| Flash point | >230 °F |
| storage temp. | 2-8°C |
| solubility | The solubility of o-phthalaldehyde is 3g/100 mL diisopropyl ether, 5g/100mL deionized water, 20g/100mL chloroform, or 20g/100mL acetone at 20°C. |
| form | powder |
| color | yellow |
| PH | 7 (53g/l, H2O, 20℃) |
| Water Solubility | soluble |
| Sensitive | Air Sensitive |
| Merck | 14,7368 |
| BRN | 878317 |
| Exposure limits | ACGIH: SL .025 mg/100 cm2; Ceiling 0.1 ppb (Skin) |
| Stability | Stable. Air sensitive. Incompatible with strong oxidizing agents, strong bases. |
| InChIKey | ZWLUXSQADUDCSB-UHFFFAOYSA-N |
| LogP | 0.99 at 30℃ |
| CAS DataBase Reference | 643-79-8(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 4P8QP9768A |
| NIST Chemistry Reference | O-phthalaldehyde(643-79-8) |
| Pesticides Freedom of Information Act (FOIA) | ortho-Phthalaldehyde |
| EPA Substance Registry System | 1,2-Benzenedicarboxaldehyde (643-79-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H314-H317-H335-H410 | |||||||||
| Precautionary statements | P260-P273-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | Xi,T,N,C | |||||||||
| Risk Statements | 36/37/38-43-34-25-50-52/53 | |||||||||
| Safety Statements | 26-28-36-45-36/37/39-61-37/39 | |||||||||
| RIDADR | UN2923 8/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TH6950000 | |||||||||
| F | 1-8-10 | |||||||||
| Autoignition Temperature | 480 °C | |||||||||
| Hazard Note | Irritant/Air Sensitive | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | Ⅱ | |||||||||
| HS Code | 29122900 | |||||||||
| Hazardous Substances Data | 643-79-8(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in Rabbit: 178 mg/kg LD50 dermal Rat > 2000 mg/kg | |||||||||
| NFPA 704 |
|
o-Phthalaldehyde price More Price(52)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.21027 | Phthaldialdehyde for synthesis | 643-79-8 | 500mg | $49.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.21027 | Phthaldialdehyde for synthesis | 643-79-8 | 10g | $130 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.21027 | Phthaldialdehyde for synthesis | 643-79-8 | 50g | $375 | 2024-03-01 | Buy |
| Sigma-Aldrich | 79760 | Phthaldialdehyde for fluorescence, ≥99.0% (HPLC) | 643-79-8 | 1g | $228 | 2024-03-01 | Buy |
| Sigma-Aldrich | 79760 | Phthaldialdehyde for fluorescence, ≥99.0% (HPLC) | 643-79-8 | 5g | $708 | 2024-03-01 | Buy |
o-Phthalaldehyde Chemical Properties,Uses,Production
Chemical Properties
o-Phthalaldehyde is a pale yellow crystalline solid.

o-Phthalaldehyde is mainly used as a high-level disinfectant (a low-temperature chemical method) for heat-sensitive medical and dental equipment such as endoscopes and thermometers; in recent years, it has gained popularity as a safe and better alternative to glutaraldehyde.
There are some researches show, pH7.5 contains the sterilizing agent of o-phthalaldehyde 0.5%, and its sterilizing power, sterilization speed, stability and toxicity all are better than glutaraldehyde, can kill mycobacterium in the 5min, the bacterium number reduces by 5 logarithmic value, and o-phthalaldehyde is very stable, tasteless in pH3~9 scopes, non-stimulated to human nose, eye mucosa, and need not activate before using, various materials are had good consistency, have tangible microbiocidal activity.
Uses
o-Phthalaldehyde can be widely used for precolumn derivatization of amino acids in HPLC separation or Capillary electrophoresis. For flow cytometric measurements of protein thiol groups.
Uses
Disinfectant. Reagent in fluorometric determination of primary amines and thiols.
Uses
Precolumn derivatization reagent for primary amines and amino acids. The fluorescent derivative can be detected by reverse-phase HPLC. The reaction requires OPA, primary amine and a sulfhydryl. In the presence of excess sulfhydryl, amines can be quantitated. In the presence of excess amine, sulfhydryls can be quantitated.
Preparation
o-Phthalaldehyde is a high-level chemical disinfectant that is commonly used for disinfection of dental and medical instruments as an alternative to glutaraldehyde, which is a known skin and respiratory sensitizer.
A variety of processes for manufacturing o-phthalaldehyde have been reported in the literature.
o-Phthalaldehyde is produced by heating pure benzaldehyde and chloroform with potassium hydroxide solution. The resulting solution is further acidified with hydrochloric acid and cooled to yield a colorless powder of o-phthalaldehyde.
It is also produced by ozonization of naphthalene in alcohol followed by catalytic hydrogenation.
Catalytic oxidation of various chemicals is also used in manufacturing o-phthalaldehyde. o-Phthalaldehyde can be manufactured by oxidation of phthalan by nitrogen monoxide in acetonitrile with N-hydroxyphthalimide as the catalyst to yield 80% to 90%.
Definition
ChEBI: A dialdehyde in which two formyl groups are attached to adjacent carbon centres on a benzene ring.
Definition
Using o-phthalaldehyde (OPA) in combination with a thiol reagent is a standard method for detecting primary amines in amino acids, peptides, and proteins. O-phthalaldehyde, in the presence of 2-mercaptoethanol, reacts with primary amines to form highly fluorescent products. Picomole quantities of amino acids, peptides, and proteins can be detected easily. o-Phthalaldehyde is five to ten times more sensitive than fluorescamine and is soluble and stable in aqueous buffers. Despite its widespread use, the exact reaction mechanism has been debated since the 1980s. Rovelli's results support the mechanism originally proposed by Sternson and Wong, in which the primary amine first reacts with OPA, followed by a reaction with the thiol to form the fluorescent isoindole product[1-2].
Synthesis Reference(s)
Journal of the American Chemical Society, 73, p. 1668, 1951 DOI: 10.1021/ja01148a076
Tetrahedron Letters, 27, p. 1793, 1986 DOI: 10.1016/S0040-4039(00)84377-4
Reactivity Profile
o-Phthalaldehyde (OPA)-amine reaction and OPA-amine-thiol reaction have been developed to effectively modify native peptides and proteins under the physiological conditions. First, OPA and its derivatives can rapidly and smoothly react with primary amine moieties in peptides and proteins to achieve native protein biconjugations. Furthermore, OPA-alkyne bifunctional linkers can be used for proteome profiling. Second, OPA-amine-thiol three-component reaction has been developed for chemoselective peptide cyclization, directly on unprotected peptides in the aqueous buffer. Moreover, this OPA-guided cyclic peptide can be further modified with the N-maleimide moiety in one pot to introduce additional functionalities[3].
Flammability and Explosibility
Not classified
Biotechnological Applications
O-phthalaldehyde(OPA) is used for precolumn derivatization of amino acids for HPLC separation and for flow cytometric measurements of protein thiol groups. Used for fluorometric determination of histamine, histidine and other amino acids. Also used for cholesterol assay in the picomole range.
Phthaldialdehyde has been used:
in the preparation of O-phthaldialdehyde reagent for analysing gentamycin content.
in the preparation of reagent for determining the degree of hydrolysis of milk proteins.
in the measurement of free amino acids of milk samples by O-phthaldialdehyde/N-acetyl-L-cysteine (OPA/NAC) assay.
in the derivatization of putrescine samples.
Potential Exposure
The primary routes of human exposure to o-phthalaldehyde are by inhalation and through the skin, which may occur through accidental or occupational exposures. Along with its increasing popularity as a chemical sterilizer, o-phthalaldehyde has many applications in analytical methods and in diagnostic kits. o-Phthalaldehyde is also used as an intermediate in the synthesis of pharmaceuticals and as a reagent in the tanning industry, hair colorings, wood treatment, and antifouling paints. o-Phthalaldehyde was approved for use as an indoor antimicrobial pesticide in 1997; however, it is no longer registered with the United States Environmental Protection Agency (USEPA) for this use.
Carcinogenicity
No information on the carcinogenicity of o-phthalaldehyde in experimental animals or humans was found in a review of the literature.
References
[1] Grazia Rovelli, Kevin R Wilson. “Elucidating the Mechanism for the Reaction of o-Phthalaldehyde with Primary Amines in the Presence of Thiols.” The Journal of Physical Chemistry B 127 14 (2023): 3257–3265.
[2] J R Benson, ;P E Hare. “O-phthalaldehyde: fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin.” Proceedings of the National Academy of Sciences of the United States of America 72 2 (1975): 619–22.
[3] “Chemical Tools for Imaging, Manipulating, and Tracking Biological Systems: Diverse Methods for Optical Imaging and Conjugation.” Methods in enzymology 1 1 (1900).
o-Phthalaldehyde Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2961 | 58 |
| Univar Solutions(China) Co., Ltd. | +8615902132654 | ivy.zhuang@univarsolutions.com | China | 654 | 58 |
| Jinan Qinmu Fine Chemical Co.,Ltd. | +8618660799346 | sales@qinmuchem.com | China | 1009 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Changzhou Zechong Biopharma Co., Ltd. | +undefined18621330623 | sales@zechongchem.com | China | 28 | 58 |
| Mainchem Co., Ltd. | -- | sarah@mainchem.com | China | 6567 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +8615350851019 | admin@86-ss.com | China | 1001 | 58 |
| Shandong Deshang Chemical Co., Ltd. | +86-0531-8875-2665 +8613153039501 | info@deshangchem.com | China | 662 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5889 | 58 |
Related articles
- Phthalaldehyde: A Versatile Compound in Modern Chemistry and Biotechnology
- Phthalaldehyde (OPA) is a pale yellow solid, it is a building block in the synthesis of heterocyclic compounds and a reagent i....
- Oct 24,2024
- o-Phthalaldehyde: derivatives and applications
- o-Phthalaldehyde and its derivatives have various applications, including as reagents, in photoresists, and as mechanically re....
- Jul 27,2023
- Introduction of Ortho-phthalaldehyde
- Based on this, a modified o-phthalaldehyde fluorometric analytical method has been established to determine ultratrace ammoni....
- Aug 26,2019
View Lastest Price from o-Phthalaldehyde manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-18 | o-Phthalaldehyde
643-79-8
|
US $0.00 / kg | 1kg | 99% | 200mt | Jinan Finer Chemical Co., Ltd | |
 |
2024-12-18 | o-Phthalaldehyde
643-79-8
|
US $65.00-60.00 / kg | 0.0010000000474974513kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-12-18 | o-Phthalaldehyde
643-79-8
|
US $85.00 / kg | 1kg | 99% | 5000kg/week | Hebei Zhuanglai Chemical Trading Co.,Ltd |
-

- o-Phthalaldehyde
643-79-8
- US $0.00 / kg
- 99%
- Jinan Finer Chemical Co., Ltd
-

- o-Phthalaldehyde
643-79-8
- US $65.00-60.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- o-Phthalaldehyde
643-79-8
- US $85.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd
643-79-8(o-Phthalaldehyde)Related Search:
1of4