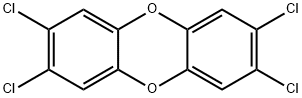
2,3,7,8-TETRACHLORODIBENZO-P-DIOXIN
- CAS No.
- 1746-01-6
- Chemical Name:
- 2,3,7,8-TETRACHLORODIBENZO-P-DIOXIN
- Synonyms
- DIOXIN;Dioxine;TCDD;2,3,7,8-TCDD;2,3,7,8-tetrachlorodibenzodioxin;Tetrachlorodibenzodioxin;tetrachlorodibenzo-p-dioxin;2,3,7,8-Tetrachlorodibenzo[b,e][1,4]dioxin;TCDBD;Dioksyny
- CBNumber:
- CB6739638
- Molecular Formula:
- C12H4Cl4O2
- Molecular Weight:
- 321.97
- MDL Number:
- MFCD00152860
- MOL File:
- 1746-01-6.mol
| Melting point | 284-287°C |
|---|---|
| Boiling point | 407.62°C (rough estimate) |
| Density | 1.6430 (estimate) |
| vapor pressure | 340 at 25 °C (Rodorf, 1985) |
| refractive index | 1.6430 (estimate) |
| Flash point | 4 °C |
| storage temp. | Refrigerator |
| solubility | Chloroform: Slightly soluble |
| form | Colorless solids or crystals |
| color | Colorless to white needles |
| Water Solubility | 0.0193ug/L(22 ºC) |
| Henry's Law Constant | 5.40 at 20 °C (approximate - calculated from water solubility and vapor pressure) |
| Exposure limits | An IDLH of 1 ppb was recommended by Schroy et al. (1985). |
| EPA Primary Drinking Water Standard | MCL:3e-008,MCLG:zero |
| CAS DataBase Reference | 1746-01-6(CAS DataBase Reference) |
| FDA UNII | DO80M48B6O |
| Proposition 65 List | 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) |
| IARC | 1 (Vol. Sup 7, 69, 100F) 2012 |
| EPA Substance Registry System | 2,3,7,8-Tetrachlorodibenzo-p-dioxin (1746-01-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H304-H315-H336-H361d-H373-H410 | |||||||||
| Precautionary statements | P210-P260-P280-P301+P310-P370+P378-P403+P235 | |||||||||
| Hazard Codes | F,Xn | |||||||||
| Risk Statements | 11-38-48/20-63-65-67 | |||||||||
| Safety Statements | 36/37-62-46 | |||||||||
| RIDADR | 2811 | |||||||||
| WGK Germany | 3 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | I | |||||||||
| Toxicity | LD50 in male, female rats (mg/kg): 0.022, 0.045 orally (Schwetz) | |||||||||
| NFPA 704 |
|
2,3,7,8-TETRACHLORODIBENZO-P-DIOXIN Chemical Properties,Uses,Production
Description
The term dioxins refers to chlorinated hydrocarbons containing
a dibenzo-p-dioxin structure (two benzene rings conjoined at
their para carbons by two oxygen molecules). The nomenclature
for chlorinated dibenzodioxins (CDD) is based on the
number and position of the chlorine molecules and include
mono- (MCDD), di- (DCDD), tri- (TrCDD), tetra- (TCDD),
penta- (PeCDD), hexa- (HxCDD), hepta- (HpCDD), and octachlorinated
(OCDD) congeners. There are 75 congeners in
total. The 2,3,7,8-tetrachlorodienzo-p-dioxin (2,3,7,8-TCDD)
congener is the most familiar congener due in part to its toxicity
in animal models, its widespread distribution and persistence
in the environment, its bioaccumulation potential, and
because most data pertain to this congener. Dioxins in pure
form are colorless solids and are formed as combustion products.
Dioxins may be formed during the combustion of organic
material in the presence of halogens, especially chlorine (and
bromine), during waste incineration and forest fires. They also
occur as contaminants in herbicides.
Dioxin was a contaminant of Agent Orange and possibly
responsible for some of the adverse health effects associated
with exposure to the defoliant. Dioxin was the poisoning agent
in a high-profile political incident in 2004. Dioxin was ultimately
identified as the cause of the disfiguring acne-like skin
(chloracne) condition suffered by Ukrainian opposition leader
Viktor Yushchenko a few months prior to the first presidential
election. The suspicion was that the dioxin was placed into
soup consumed by Mr Yushchenko. The acne-like skin condition
is a recognized hallmark of dioxin poisoning in humans.
The actual intake of dioxin in this incident is unknown.
Chemical Properties
Tetrachlorodibenzo-p-dioxin is a white, needleshaped, crystalline solid.
Chemical Properties
White Solid
Uses
A toxic polychlorinated dibenzo-p-dioxin detected in domestic meat and poultry.
Uses
With the exception of its use in research, it is ironic that there are no known uses for any of the dioxins. They are unintended byproducts of chemical manufacturing and combustion. There is no commercial manufacture of these compounds. The limited production of dioxins for use in research involves production by condensation of polychlorophenol or, for a specific dioxin, by chlorination of the parent dibenzo-p-dioxin.
Production Methods
2,3,7,8-TCDD is not commercially produced except for its
use as a research chemical. 2,3,7,8-TCDDis a contaminant of
2,4,5-trichlorophenol (2,4,5-TCP), the herbicide 2-(2,4,5-
trichlorophenoxy)propionic acid [Silvex], the herbicide
2,4,5-trichlorophenoxyacetic acid (2,4,5-T), the wood
preservative pentachlorophenol, hexachlorophene,
hexachlorobenzene, and polychlorodiphenyl ethers.
2,3,7,8-TCDD is also produced by incineration of municipal,
hospital, and toxic wastes and sludges and wood that contain
chlorinated compounds and materials, of polyvinylchloride
containing plastics, by paper and pulp bleaching, during
PCB electrical transformer fires, during the hot processes of dye and pigment manufacturing, and smelter emissions.
It is not imported into the United States.
The major source of 2,3,7,8-TCDDwas in the manufacture
of 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), which was
introduced in the late 1940s. Prior to 1965, commercial 2,4,5-T
contained up to 30 mg/kg (ppm) 2,3,7,8-TCDD but it was
reduced to 0.01 ppm in the mid-1980s. Its use peaked in the
1970s, and has been phased out in Europe and the United
States. The levels of 2,3,7,8-TCDD in the Vietnam War
herbicide Agent Orange (1:1 mixture of the n-butyl esters
of 2,4,5-T and (2,4-dichlorophenoxy)acetic acid (2,4-D))
varied considerably from 0.02 to 47 mg/kg (ppm). In the
1960s, the level of 2,3,7,8-TCDD could have been as high as
100 mg/kg in Agent Orange. In the 1980s, all producers
claimed that 2,3,7,8-TCDD concentrations were less than
0.1 mg/kg.
2,3,7,8-TCDD and other PCDDs are formed by hot industrial,
thermal, and photochemical processes that involve
chlorinated organics.
Definition
ChEBI: 2,3,7,8-tetrachlorodibenzodioxine is a polychlorinated dibenzodioxine.
General Description
White crystals or tan crystalline powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
2,3,7,8-TETRACHLORODIBENZO-P-DIOXIN reaacts when exposed to ultraviolet light in solution in isooctane or n-octanol. Undergoes catalytic perchlorination .
Health Hazard
Chlorinated dibenzo-p-dioxins
(CDDs) cause chloracne, may cause hepatotoxicity,
immunotoxicity, reproductive toxicity,
developmental toxicity, and central nervous
system toxicity, and are considered to be a
human carcinogen.
The most obvious health effect in humans
for exposure to CDDs is chloracne, a severe
skin disease characterized by follicular hyperkeratosis
(comedones) occurring with or without
cysts and pustules.2–4 Unlike adolescent acne,
chloracne may affect almost every follicle in an
involved area, and it may be more disfiguring
than adolescent acne.
Fire Hazard
Literature sources indicate that 2,3,7,8-TETRACHLORODIBENZO-P-DIOXIN is nonflammable.
Pharmacology
TCDD and other chlorinated dibenzodioxins, dibenzofurans, and planar PCBs are thought to operate through a common mechanism. For humans and rodents, there is an initial binding to the aryl hydrocarbon (Ah) receptor. Binding to the receptor is a necessary (but not sufficient) event for the biological response. TCDD induces many responses, including induction of gene expression, altered metabolism, altered cell growth and differentiation, and disruption of steroid hormone and growth factor signal transduction pathways. The very diversity of tissue-selective and species-selective responses elicited by TCDD requires that the receptor (Ah) is part of a multicomponent system, and it is unlikely that the differences in dose-response are related solely to differences in Ah receptor concentrations or affinities in various species or tissues (29). It is considered that there is an inducible protein-binding site in the liver (30,31) known as CYP1A1 (30–34) because TCDD was not sequestered in the liver of transgenic mice that lack P450 1A2 gene.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. One of the most toxic synthetic chemicals. A deadly experimental poison by ingestion, skin contact, and intraperitoneal routes. Human systemic effects by skin contact: allergic dermatitis. Experimental reproductive effects. Human mutation data reported. An eye irritant. TCDD is the most toxic member of the 75 dioxins. It causes death in rats by hepatic cell necrosis. Death can follow a lethal dose by weeks. Acute and subacute exposure result in wasting, hepatic necrosis, thymic atrophy, hemorrhage, lymphoid depletion, chloracne. A by-product of the manufacture of polychlorinated phenols. It is found at low levels in 2,4,5-T, 2,4,5-trichlorophenol, and hexachlorophene. It is also formed during various combustion processes. Incineration of chemical wastes, including chlorophenols, chlorinated benzenes, and biphenyl ethers, may result in the presence of TCDD in flue gases, fly ash, and soot particles. It is immobile in contaminated soil and may be retained for years. TCDD has the potential for bio-accumulation in animals. An accident in Seveso, Italy, and inadvertent soil contamination in Mmouri have resulted in abandonment of the contaminated areas. When heated to decomposition it emits toxic fumes of Cl-.
Potential Exposure
TCDD is primarilly a research chemical. As noted above, TCDD is an inadvertent contaminant in herbicide precursors and thus in the herbicides themselves. It is also formed during various combustion processes including the incineration of chemical wastes (chlorophenols, chlorinated benzenes, and biphenyl ethers). It may be found in flue gases, fly ash, and soot particles. It is highly persistent in soil, and contamination may be retained for years. TCDD is the most toxic of all the dioxins, and has the potential for bio-accumulation in animals. Thus, it is applied in herbicide formulations, but is not used per se. It has been estimated that approximately 2 million acres in the United States have been treated for weed control on one or more occasions with approximately 15 million pounds of TCDD contaminated 2,4,5,-T, 2,4,-D, or combinations of the two.
Carcinogenicity
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans, both epidemiological and on the mechanism of carcinogenesis. TCDD was first listed in the Second Annual Report on Carcinogens as reasonably anticipated to be a human carcinogen. Subsequently, a number of studies were published that examined cancer in human populations exposed to TCDD occupationally or through industrial accidents. A concerted research effort examined the molecular and cellular events that occur in tissues of humans and animals exposed to TCDD. Based on the new information, the listing was revised to known to be a human carcinogen in the January 2001 addendum to the Ninth Report on Carcinogens.
Source
Although not produced commercially, TCDD is formed as a by-product in the synthesis
of 2,4,5-trichlorophenol. TCDD was found in 85% of soil samples obtained from a trichlorophenol
manufacturing site. Concentrations ranged from approximately 20 ng/kg to 600 g/kg (Van Ness et
al., 1980). TCDD may be present in the herbicide 2,4-D which contains a mixture of dichloro-,
trichloro-, and tetrachlorodioxins. TCDD is commonly found as a contaminant associated with
pulp and paper mills (Boddington, 1990). In addition, during the manufacture of 2,4,5-T and
silvex from trichlorophenol, TCDD was found at concentrations averaging 20 parts per billion
(Newton and Snyder, 1978).
TCDD is unintentionally formed during the combustion of domestic and industrial waste
(Czuczwa and Hites, 1984, 1986) and bleaching of paper pulp by chlorine compounds (Buser et
al., 1989; Swanson et al., 1988).
Drinking water standard (final): MCLG: zero; MCL: 3 x 10-5 μg/L (U.S. EPA, 2000). In
Canada, the Ontario Ministry of Environment has established an Interim Drinking Water
Objective of 10 parts per quadrillion (Boddington, 1990). In addition, the U.S. EPA (2000)
recommended a DWEL of 4 x 10-5 μg/L.
Environmental Fate
Biological. After a 30-d incubation period, the white rot fungus Phanerochaete chrysosporium
was capable of oxidizing TCDD to carbon dioxide. Mineralization began between the third and
sixth day of incubation. The production of carbon dioxide was highest between 3 to 18 d of
incubation, after which the rate of a carbon dioxide produced decreased until the 30th day. It was
suggested that the metabolism of TCDD and other compounds, including p,p′-DDT,
benzo[a]pyrene, and lindane, was dependent on the extracellular lignin-degrading enzyme system
of this fungus (Bumpus et al., 1985).
A half-life of 418 d was calculated based on die away test data (Kearney et al., 1971).
In a laboratory sediment-water system incubated under anaerobic conditions, the half-life of
TCDD was 500 to 600 d (Ward and Matsumura, 1978).
Soil. In shallow and deep soils, reported half-lives were 10 and 100 yr, respectively (Nauman
and Schaum, 1987). Due to its low aqueous solubility, TCDD will not undergo significant
leaching by runoff (Helling et al., 1973).
Surface Water. Plimmer et al. (1973) reported that the photolysis half-life of TCDD in a
methanol solution exposed to sunlight was 3 h. Volatilization half-lives of 32 and 16 d were
reported for lakes and rivers, respectively (Podoll et al., 1986).
Photolytic. Pure TCDD did not photolyze under UV light. However, in aqueous solutions
containing cationic (1-hexadecylpyridinium chloride), anionic (sodium dodecyl sulfate), and
nonionic (methanol) surfactants, TCDD decomposed into the end product tentatively identified as
2-phenoxyphenol. The times required for total TCDD decomposition using the cationic, anionic,
and nonionic solutions were 4, 8, and 16 h, respectively (Botré et al., 1978). TCDD photodegrades
rapidly in alcoholic solutions by reductive dechlorination. In water, however, the reaction was
very slow (Crosby et al., 1973). In an earlier study, Crosby et al. (1971) reported a photolytic halflife
of 14 d when TCDD in distilled water was exposed to sunlight. The major photodegradative
pathway of TCDD involves a replacement of the chlorine atom by a hydrogen atom. The proposed
degradative pathway is TCDD to 2,7,8-trichlorodibenzo[b,e][1,4]dioxin to 2,7-dichlorodibenzo-
[b,e][1,4]dioxin to 2-chlorodibenzo[b,e][1,4]dioxin to dibenzo[b,e][1,4]dioxin to 2-hydroxydiphenyl
ether, which undergoes polymerization (Makino et al., 1992).
Chemical/Physical. TCDD was dehalogenated by a solution of poly(ethylene glycol), potassium
carbonate, and sodium peroxide. After 2 h at 85 °C, >99.9% of the applied TCDD decomposed.
Chemical intermediates identified include tri-, di-, and chloro[b,e]dibenzo[1,4]dioxin, dibenzodioxin,
hydrogen, carbon monoxide, methane, ethylene, and acetylene (Tundo et al., 1985).
TCDD will not hydrolyze to any reasonable extent (Kollig, 1993).
Metabolism
Absorption. TCDD is retained in all tissues. The
highest retention is in fat and liver.
Penetration values into human skin are low. For example,
a dose of 6.5 ng/cm2 in acetone gave a rate of 5 g/cm2/h.
Transfer to the fetus has been observed (43).
Absorption rates after single dose in the diet were 50
to 70–90% (44–48). Rates in rats were lower (50–60%) when administered in the diet for more than 6 weeks (49),
compared with a single-dose absorption rate of 70% (46).
Distribution. The major storage sites are liver and
adipose tissue. The skin can act as an important storage
site, and high concentrations can also be found in the
adrenals (1). After one day of exposure for rats, mice,
hamsters and guinea pigs, 25–70% of the dose was stored
in the liver (41).
Excretion. Excretion is mostly fecal. Breast milk can be
a route of elimination. Whole body half-lives were from
17 to 31 days in rat studies (46–52). Mice had lower halflives
(53,54). Female rhesus monkeys with four years of
dietary exposure had a longer half-life (391 days) (55,56).
These half-lives are very fast considering human half-lives
of 5.8–11.3 years (cited earlier).
Solubility in organics
Acetone (110 mg/L), benzene (570 mg/L), chlorobenzene (720 mg/L) chloroform (370 mg/L), o-dichlorobenzene (1,400 mg/L), methanol (10 mg/L) and octanol (48–50 mg/L) (Crummett and Stehl, 1973; Arthur and Frea, 1989); benzene (570 mg/L), tetrachloroethene (680 mg/L), lard oil (40 mg/L), hexane (280 mg/L) (quoted, Keith and Walters, 1992).
Solubility in water
Acetone (110 mg/L), benzene (570 mg/L), chlorobenzene (720 mg/L) chloroform (370 mg/L), o-dichlorobenzene (1,400 mg/L), methanol (10 mg/L) and octanol (48–50 mg/L) (Crummett and Stehl, 1973; Arthur and Frea, 1989); benzene (570 mg/L), tetrachloroethene (680 mg/L), lard oil (40 mg/L), hexane (280 mg/L) (quoted, Keith and Walters, 1992).
Toxicity evaluation
Mortality
Mortality occurs after several days to weeks of
exposure. Toxic effects observed in all animal species
are progressive loss of body weight, reduced intake
of food, atrophy of the thymus, gastrointestinal
hemorrhage, and delayed lethality (17,18).
Skin
Skin effects are exhibited by humans and non human
primates and
are not modeled by laboratory animals, although some experimentation has been performed with
hairless mice.
Cachexia
All mammalian species show body weight loss and
reduced intake of food. Studies by Pohjanvirta and
Tuomisto (19) indicated that TCDD may suppress
the formation of hunger-related signals. A serotonergic
mechanism was proposed because of increased
levels of tryptophan and its metabolites, serotonin
and 5-hydroxyindoleacetic acid, in blood and brain.
Endocrine effects
A variety of hormone systems are involved with exposure
to TCDD, specifically, sex steroids, corticosteroids,
and thyroid hormones. A target organ for
TCDD is the pituitary gland where normal feedback
mechanisms are disrupted (20).
Immunological Effects
Immunological effects are observed in mammals but
are probably without relevance for humans.
Incompatibilities
Decomposes in ultraviolet (UV) light.
2,3,7,8-TETRACHLORODIBENZO-P-DIOXIN Preparation Products And Raw materials
Raw materials
Preparation Products
1746-01-6(2,3,7,8-TETRACHLORODIBENZO-P-DIOXIN)Related Search:
1of4