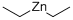Diethylzinc
- CAS No.
- 557-20-0
- Chemical Name:
- Diethylzinc
- Synonyms
- Et2Zn;DEZ;DEZn;CHEMR23;diethyl-zin;diethylzine;Diethylzinc,elec.gr.(99.999%-Zn)PURATREM;(C2H5)2Zn;ZINC ETHYL;Zinc ethide
- CBNumber:
- CB7149427
- Molecular Formula:
- C4H10Zn
- Molecular Weight:
- 123.51
- MDL Number:
- MFCD00009021
- MOL File:
- 557-20-0.mol
| Melting point | −28 °C(lit.) |
|---|---|
| Boiling point | 98 °C |
| Density | 1.205 g/mL at 25 °C(lit.) |
| vapor pressure | 16hPa at 20℃ |
| refractive index |
n |
| Flash point | 45 °F |
| storage temp. | 0-6°C |
| solubility | reacts with H2O; msc ethyl ether,petroleum ether, benzene |
| form | Solution |
| Specific Gravity | 0.740 |
| color | Slightly turbid light brown-gray |
| Water Solubility | REACTS VIOLENTLY |
| Sensitive | Air & Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Merck | 14,3131 |
| BRN | 3587207 |
| Dielectric constant | 2.5(20℃) |
| CAS DataBase Reference | 557-20-0(CAS DataBase Reference) |
| FDA UNII | S0W5NQH7C6 |
| NIST Chemistry Reference | Diethylzinc(557-20-0) |
| EPA Substance Registry System | Zinc, diethyl- (557-20-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |      GHS02,GHS05,GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H250-H260-H304-H314-H336-H361d-H373-H411 | |||||||||
| Precautionary statements | P210-P231+P232-P280-P301+P330+P331-P303+P361+P353-P304+P340+P310-P305+P351+P338-P370+P378 | |||||||||
| Hazard Codes | F,Xn,N,C | |||||||||
| Risk Statements | 14-17-34-50/53-67-65-62-48/20-11-51/53-14/15-63 | |||||||||
| Safety Statements | 26-45-61-62-8-36/37/39-16-60-43 | |||||||||
| RIDADR | UN 3399 4.3/PG 1 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | ZH2077777 | |||||||||
| F | 10-23 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 4.3 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 29319090 | |||||||||
| NFPA 704 |
|
Diethylzinc price More Price(32)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 220809 | Diethylzinc solution 15 wt. % in toluene | 557-20-0 | 100g | $181 | 2024-03-01 | Buy |
| Sigma-Aldrich | 220809 | Diethylzinc solution 15 wt. % in toluene | 557-20-0 | 800g | $866 | 2024-03-01 | Buy |
| TCI Chemical | D3214 | Diethylzinc (ca. 17% in Hexane, ca. 1mol/L) | 557-20-0 | 100mL | $110 | 2024-03-01 | Buy |
| TCI Chemical | D3214 | Diethylzinc (ca. 17% in Hexane, ca. 1mol/L) | 557-20-0 | 500mL | $222 | 2024-03-01 | Buy |
| Alfa Aesar | 044880 | Diethylzinc, nominally 15% w/w in hexanes, packaged under Argon in resealable ChemSeal? bottles | 557-20-0 | 100g | $120 | 2021-12-16 | Buy |
Diethylzinc Chemical Properties,Uses,Production
Description
Diethyl zinc is an organometal compound and is a dangerous fire hazard. It spontaneously ignites in air and reacts violently with water, releasing flammable vapors and heat. It is a colorless, pyrophoric liquid with a specific gravity of 1.2, which is heavier than water, so it will sink to the bottom. It decomposes explosively at 248°F (120°C). It has a boiling point of 243°F (117°C), a flash point of ?20°F (?28°C), and a melting point of ?18°F (?27°C). The four-digit UN identification number is 1366. The NFPA 704 designation is health 3, flammability 4, and reactivity 3. The white space at the bottom of the diamond has a W with a slash through it to indicate water reactivity. Primary uses of diethyl zinc are in the polymerization of olefins, high-energy aircraft, and missile fuel and in the production of ethyl mercuric chloride.
Chemical Properties
Clear colorless solution. M.p. -30°C, b.p. (760 mm.) 117.6°C,(30 mm.) 27°C, (4 mm.) 0°C; d (20°C) 1.207, (8°C) 1.245. Resistant to CO3; ignites in air. Decomposes extremely violently in H3O, forming Zn(OH)a and C3H8. Soluble in ether.
Uses
Diethyl zinc is used in organic synthesis. It isalso used in preservation of archival papers.
Uses
Diethylzinc solution can be used in the synthesis of:
- Bis(pyridylpyrrolyl)zinc luminescent complexes.
- A versatile building block, 5-(ketoaryl)thiazole.
- ZnxCd1-xSe nanocrystals having high luminescence properties.
Uses
In organic synthesis; in preservation on archival papers.
Definition
ChEBI: Diethylzinc is a dialkylzinc compound.
General Description
Diethylzinc is a pyrophoric liquid with a garlic-like odor. Diethylzinc is stable when Diethylzinc is shipped in sealed tubes with carbon dioxide. Diethylzinc may decompose violently in water and ignite spontaneously with air. Diethylzinc is toxic by ingestion. If exposed to heat or flame, containers of Diethylzinc may explode. Diethylzinc is used as an aircraft fuel.
Air & Water Reactions
Highly flammable. Ignites in air with a blue flame giving off a peculiar garlic-like odor, [Merck, 11th ed., 1989]. Diethyl zinc is spontaneously flammable in air, [Douda(1966)]. Reacts violently with water to form flammable ethane gas, [Brauer(1965)].
Reactivity Profile
Diethylzinc is pyrophoric in air, Diethylzinc ignites instantaneously. Diethylzinc reacts explosively with alcohols (methanol, ethanol), bromine, chlorine or liquefied sulfur dioxide [Houben-Weyl, 1973, 13.2a, p. 855, 757, 709]. Reaction with water, nitro compounds, arsenic trichloride, phosphorus trichloride is violent [Bretherick, 5th ed., 1995, p. 587].
Health Hazard
Inhalation of mist or vapor causes immediate irritation of nose and throat; excessive or prolonged inhalation of fumes from ignition or decomposition may cause ``metal fume fever'' (sore throat, headache, fever, chills, nausea, vomiting, muscular aches, perspiration, constricting sensation in lungs, weakness, sometimes prostration); symptoms usually last 12-24 hrs., with complete recovery in 24-48 hrs. Eyes are immediately and severely irritated on contact with liquid, vapor, or dilute solution; without thorough irrigation, cornea may be permanently damaged. Moisture in skin combines with chemical to cause thermal and acid burns; tissue may be scarred without prompt treatment. Ingestion is unlikely but would cause immediate burns at site of contact; pain, nausea, vomiting, cramps, and diarrhea may follow; if untreated, tissue may become ulcerated.
Health Hazard
Being moisture sensitive, any accidental contactof the pure liquid or its concentratedsolution with the skin can cause a severeburn.
Fire Hazard
Diethylzinc ignites spontaneously in air, burning with a blue flame. Reactions with water and lower alcohols can be violent.Violent reactions can occur with halogens, halogenated hydrocarbons, nitroorganics, oxidizers, sulfur dioxide, and chlorides of phosphorus, arsenic, and antimony. With the latter compounds, diethylzinc forms pyrophoric triethylphosphine, triethyl arsine, and triethylstibine, respectively.
Flammability and Explosibility
Pyrophoric
Safety Profile
Presumed to be a poison. Ignites spontaneously in air. Dangerously flammable by spontaneous chemical reaction in air, or with oxidzing materials. A dangerous explosion hazard Explosive reaction with alkenes + diodomethane, sulfur dioxide. Reacts violently with bromine, water, nitro compounds. Igmtes on contact with air, ozone, methanol, or hydrazine. Reacts violently with nonmetal halides (e.g., arsenic trichloride or phosphorus trichloride) to produce pyrophoric triethyl arsine or triethyl phosphine. To fight fire, do not use water, foam, or halogenated extinguishing agents. Use dry materials, such as graphite, sand, etc. When heated to decomposition it emits toxic fumes of ZnO. See also ZINC COMPOUNDS.
Synthesis
Diethylzinc is obtained by the reaction of Zn and C2H5Ⅰ to produce C2H5ZnⅠ, which is then distilled under inert gas conditions.
Zn + C2H5Ⅰ = C2H5ZnⅠ; 2C2H5ZnⅠ = Zn(C2H5)2+ ZnⅠ2.
Potential Exposure
Diethyl zinc is used in organic syntheses; as a catalyst in the manufacture of olefin polymers andas a high-energy aircraft and missile fuel.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
storage
Color Code—Red Stripe: Flammability Hazard:Store separately from all other flammable materials. Priorto working with diethyl zinc you should be trained on itsproper handling and storage. Before entering confined spacewhere diethyl zinc may be present, check to make sure thatan explosive concentration does not exist. Diethyl zinc mustbe stored to avoid contact with water, chlorine, hydrazine,and oxidizers since violent reactions occur. Store in sealedtubes or cylinders under a dry, inert gas blanket or in anevacuated system. Shade from radiant heat and protect fromrain. Metal containers involving the transfer of=gallons ormore of diethyl zinc should be grounded and bonded.Wherever diethyl zinc is used, handled, manufactured, orstored, use explosion proof electrical equipment and fittings. Use only nonsparking tools and equipment, especiallywhen opening and closing containers of this chemical.Sources of ignition, such as smoking and open flames, areprohibited where this chemical is used, handled, or stored ina manner that could create a potential fire or explosionhazard.
Shipping
This compound requires a shipping label of“SPONTANEOUSLY COMBUSTIBLE.” It falls in Hazard4.2 and Packing Group I.
Incompatibilities
Ignites spontaneously on contact with airor strong oxidizers. Explosive decomposition at 245°F/120℃. Violent reaction with hydrazine, sulfur dioxide,halogens, some alcohols, ozone; possible fire and explosions. Contact with water forms ethane gas.
Diethylzinc Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
| Changzhou Xuanming Pharmaceutical Technology Co., Ltd. | +86-519-85525359 +86-15995072465 | sales@xuanmingchem.com | China | 914 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29884 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9636 | 58 |
View Lastest Price from Diethylzinc manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | Diethylzinc
557-20-0
|
US $80.00-180.00 / ML | 1ML | 99$ | 10000 | Changzhou Xuanming Pharmaceutical Technology Co., Ltd. | |
 |
2023-08-26 | Diethylzinc
557-20-0
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2021-10-12 | Diethylzinc
557-20-0
|
US $9.90 / KG | 1KG | 99% | 5tons | Hebei Weibang Biotechnology Co., Ltd |
-

- Diethylzinc
557-20-0
- US $80.00-180.00 / ML
- 99$
- Changzhou Xuanming Pharmaceutical Technology Co., Ltd.
-

- Diethylzinc
557-20-0
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Diethylzinc
557-20-0
- US $9.90 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd
557-20-0(Diethylzinc)Related Search:
1of4