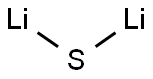
Lithium sulfide
- CAS No.
- 12136-58-2
- Chemical Name:
- Lithium sulfide
- Synonyms
- Thiobislithium;LITHIUMSULPHIDE;LITHIUM SULFIDE;dilithiumsulfide;Lithium sulfide 99%;lithiumsulfide(li2s);dilithiummonosulfide;Lithium sulphide, 99%+;(lithiosulfanyl)lithium;Lithium sulphide, 99.9%
- CBNumber:
- CB7245998
- Molecular Formula:
- Li2S
Lewis structure

- Molecular Weight:
- 45.95
- MDL Number:
- MFCD00011085
- MOL File:
- 12136-58-2.mol
- MSDS File:
- SDS
| Melting point | >900°C | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density | 1.66 g/mL at 25 °C (lit.) | ||||||||||||||
| storage temp. | 2-8°C | ||||||||||||||
| form | Powder | ||||||||||||||
| Specific Gravity | 1.66 | ||||||||||||||
| color | Yellow | ||||||||||||||
| Water Solubility | soluble in water and ethanol | ||||||||||||||
| Sensitive | Moisture Sensitive | ||||||||||||||
| Crystal Structure | Reverse CaF2 type | ||||||||||||||
| crystal system | Cube | ||||||||||||||
| Space group | Fm3m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| InChI | InChI=1S/2Li.S | ||||||||||||||
| InChIKey | ZWDBUTFCWLVLCQ-UHFFFAOYSA-N | ||||||||||||||
| SMILES | S([Li])[Li] | ||||||||||||||
| CAS DataBase Reference | 12136-58-2(CAS DataBase Reference) | ||||||||||||||
| EWG's Food Scores | 1-2 | ||||||||||||||
| FDA UNII | SW6C51V9JZ | ||||||||||||||
| EPA Substance Registry System | Lithium sulfide (Li2S) (12136-58-2) | ||||||||||||||
| UNSPSC Code | 12352300 | ||||||||||||||
| NACRES | NA.23 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H314 | |||||||||
| Precautionary statements | P260-P270-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 22-31-34 | |||||||||
| Safety Statements | 26-36/37/39-45 | |||||||||
| RIDADR | UN 2923 8/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | OJ6439500 | |||||||||
| F | 8-10-13 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| NFPA 704 |
|
Lithium sulfide price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 213241 | Lithium sulfide 99.98% trace metals basis | 12136-58-2 | 5g | $147 | 2024-03-01 | Buy |
| Sigma-Aldrich | 213241 | Lithium sulfide 99.98% trace metals basis | 12136-58-2 | 10g | $257 | 2024-03-01 | Buy |
| Alfa Aesar | 012839 | Lithium sulfide, 99.9% (metals basis) | 12136-58-2 | 2g | $52 | 2023-06-20 | Buy |
| Alfa Aesar | 012839 | Lithium sulfide, 99.9% (metals basis) | 12136-58-2 | 10g | $160 | 2023-06-20 | Buy |
| Strem Chemicals | 93-0378 | Lithium sulfide, 98% (99.9%-Li) | 12136-58-2 | 5g | $102 | 2024-03-01 | Buy |
Lithium sulfide Chemical Properties,Uses,Production
Chemical Properties
Lithium sulfide is the inorganic compound with the formula Li2S. It crystallizes in the antifluorite motif, described as the salt (Li+)2S2−. It forms a solid yellow-white deliquescent powder. In air, it easily hydrolyses to release hydrogen sulfide (rotten egg odor) Lithium sulfide is prepared by treating lithium with sulfur. This reaction is conveniently conducted in anhydrous ammonia. The THF-soluble triethylborane adduct of lithium sulfide can be generated using superhydride.

Lithium sulfide (Li2S) is considered the promising cathode material for its high theoretical capacity, high melting point, affordable volume expansion, and lithium composition.
Physical properties
Lithium Sulfide, Li2S, is an anti-fluorite semiconductor with a band-gap of 3.865 eV. It also has exactly the same valence electron count, Ne, and atomic number, Z, as magnesium diboride, MgB2. Both have almost the same formula weight. This qualifies Li2S as a magnesium-diboride like material. Li2S passes the same computational material specific test for superconductivity as MgB2.
Lithium sulfide is a much studied material, though never tested for superconductivity. Li2S can exist in two forms: orthorhombic and cubic. The orthorhombic form belongs to space group Pmnb and has dimensions: a = 3.808Å; b = 6.311Å; c = 7.262Å. It has density of 1.75g/cm3 . The cubic version has density of 1.63g/ cm3, belongs to space group Fm-3m and has cubic dimensions 4.046Å. The electronic structure and density of states indicate that cubic Li2S is an indirect band-gap semiconductor with a band gap of 3.865 eV. Lithium sulfide melts between 900 – 975 degrees centigrade.
Uses
Lithium sulfide (Li2S) is a product specially designed for the use in high performance batteries which can be either applied as electrode material or as precursor for solid electrolytes. It as an electrode material not only has high capacity but also overcomes many problems caused by pure sulfur electrodes.
Lithium sulfide is an anti fluorite semiconductor (bandgap 3.865eV). It exists in orthorhombic and cubic structures. The densities of the orthorhombic and cubic structures are 1.75g/cm3 and 1.63g/cm3 respectively.
Lithium sulfide has been studied as a MgB2- like superconductor. It is also used as a cathode material in rechargeable lithium-sulfur batteries.
Uses
Lithium sulfide has been studied as a MgB2- like superconductor. It is also used as a cathode material in rechargeable lithium-sulfur batteries.
Preparation
Lithium sulfide, Li2S, is formed in the reaction of lithium with sulfur in liquid ammonia, by the decomposition of the ethanol adduct of lithium hydrogen sulfide with lithium ethanolate, and, more recently, by the reaction of hydrogen sulfide with lithium amylate to yield lithium hydrogen sulfide, LiSH, which is thermally decomposed in a vacuum to yield the sulfide. A very high quality anhydrous lithium sulfide may be prepared by the reaction of lithium metal and hydrogen sulfide in tetrahydrofuran if care is taken to exclude water. The reaction product is filtered from the reaction medium, and it is vacuum dried to remove tetrahydrofuran and to decompose the small amount of lithium hydrogen sulfide which forms. Lithium sulfide is reported to have an antifluorite structure. Lithium sulfide is readily hydrolyzed, even by water in the air, yielding hydrogen sulfide. The sulfide also reacts with sulfur to form a variety of polysulfides.
General Description
Lithium sulfide is an anti fluorite semiconductor (bandgap 3.865eV). It exists in orthorhombic and cubic structures. The densities of the orthorhombic and cubic structures are 1.75g/cm3 and 1.63g/cm3 respectively.
reaction suitability
reagent type: catalyst
core: lithium
Toxicology
Large doses of lithium ion have caused dizziness and prostration, and can cause kidney damage if sodium intake is limited. Dehydration, weight loss, dermatological effects, and thyroid disturbances have been reported. Central nervous system effects that include slurred speech, blurred vision, sensory loss, ataxia, and convulsions may occur. Diarrhea, vomiting, and neuromuscular effects such as tremor, clonus, and hyperactive reflexes may occur as a result of repeated exposure to lithium ion.
Lithium sulfide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531153977 | allison@yan-xi.com | China | 5854 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20235 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21628 | 55 |
| Hubei XinRunde Chemical Co., Ltd. | +8615102730682 | bruce@xrdchem.cn | CHINA | 566 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29859 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| TopScience Biochemical | 00852-68527855 | info@itopbiochem.com | China Hong Kong | 902 | 58 |
| Hebei Binshare New Material Co. Ltd | +8618633865755 | china01@hbbinshare.com | CHINA | 955 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34563 | 58 |
Related Qustion
- Q:Is Lithium sulfide an ionic or covalent compound?
- A:Lithium Sulfide (sulphide) is an ionic compound.
- Dec 10,2024
View Lastest Price from Lithium sulfide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-07 | Lithium sulfide
12136-58-2
|
US $1000.00-8000000.00 / kg | 1kg | 99.9% | 1T | Great Innovation (Shanghai) Pharma Co., Ltd., | |
 |
2025-04-04 | Lithium sulfide
12136-58-2
|
US $0.00-0.00 / KG | 1KG | 98% | 1ton | Henan Aochuang Chemical Co.,Ltd. | |
 |
2025-03-07 | Lithium sulfide
12136-58-2
|
US $0.00 / KG | 1KG | 99.9%/99.99% | 100KG/Month | Hebei Yanxi Chemical Co., Ltd. |
-

- Lithium sulfide
12136-58-2
- US $1000.00-8000000.00 / kg
- 99.9%
- Great Innovation (Shanghai) Pharma Co., Ltd.,
-
- Lithium sulfide
12136-58-2
- US $0.00-0.00 / KG
- 98%
- Henan Aochuang Chemical Co.,Ltd.
-

- Lithium sulfide
12136-58-2
- US $0.00 / KG
- 99.9%/99.99%
- Hebei Yanxi Chemical Co., Ltd.
12136-58-2(Lithium sulfide)Related Search:
1of4